当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
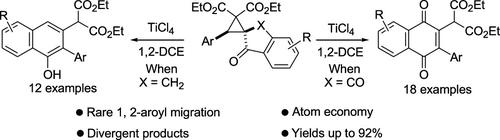
Intramolecular 1,2-Aroyl Migration in Spiro Donor–Acceptor Cyclopropanes: Formation of 1,4-Naphthoquinones and 1-Naphthols as Ring-Expansion Products
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-09 , DOI: 10.1021/acs.joc.3c02671 Franklin Leslin Daniel 1 , Kannupal Srinivasan 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-09 , DOI: 10.1021/acs.joc.3c02671 Franklin Leslin Daniel 1 , Kannupal Srinivasan 1
Affiliation
|
Most of the known rearrangement reactions of donor–acceptor cyclopropanes (DACs) involve the migration of cationic carbon atom to anionic carbon or heteroatoms in 1,3- or 1,4-positions. In the present work, we observed that spiro DACs based on 1,3-indanedione or 1-indanone moiety undergo intramolecular 1,2-aroyl migration when treated with titanium(IV) chloride to afford 1,4-naphthoquinones and α-naphthols readily. The reactions take place through the formation of putative 1,3-dipolar intermediates, followed by cleavage and migration of the aroyl group to the adjacent carbon to afford the ring-expansion products.
中文翻译:

螺环供体-受体环丙烷中的分子内 1,2-芳酰基迁移:形成 1,4-萘醌和 1-萘酚作为扩环产物
大多数已知的供体-受体环丙烷 (DAC) 重排反应涉及阳离子碳原子迁移到 1,3- 或 1,4- 位的阴离子碳或杂原子。在目前的工作中,我们观察到基于 1,3-茚满二酮或 1-茚满酮部分的螺环 DAC 在用氯化钛(IV)处理时会发生分子内 1,2-芳酰基迁移,从而很容易地生成 1,4-萘醌和 α-萘酚。该反应通过形成推定的 1,3-偶极中间体,然后芳酰基裂解并迁移至相邻的碳以提供环扩展产物而发生。
更新日期:2024-04-09
中文翻译:

螺环供体-受体环丙烷中的分子内 1,2-芳酰基迁移:形成 1,4-萘醌和 1-萘酚作为扩环产物
大多数已知的供体-受体环丙烷 (DAC) 重排反应涉及阳离子碳原子迁移到 1,3- 或 1,4- 位的阴离子碳或杂原子。在目前的工作中,我们观察到基于 1,3-茚满二酮或 1-茚满酮部分的螺环 DAC 在用氯化钛(IV)处理时会发生分子内 1,2-芳酰基迁移,从而很容易地生成 1,4-萘醌和 α-萘酚。该反应通过形成推定的 1,3-偶极中间体,然后芳酰基裂解并迁移至相邻的碳以提供环扩展产物而发生。



























 京公网安备 11010802027423号
京公网安备 11010802027423号