当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient, rapid, and high‐yield synthesis of aryl Schiff base derivatives and their in vitro and in silico inhibition studies of hCA I, hCA II, AChE, and BuChE
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2024-04-09 , DOI: 10.1002/ardp.202300266 Musa Özil 1 , Halis T. Balaydın 2 , Berna Dogan 3, 4 , Murat Şentürk 5 , Serdar Durdagi 6, 7
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2024-04-09 , DOI: 10.1002/ardp.202300266 Musa Özil 1 , Halis T. Balaydın 2 , Berna Dogan 3, 4 , Murat Şentürk 5 , Serdar Durdagi 6, 7
Affiliation
|
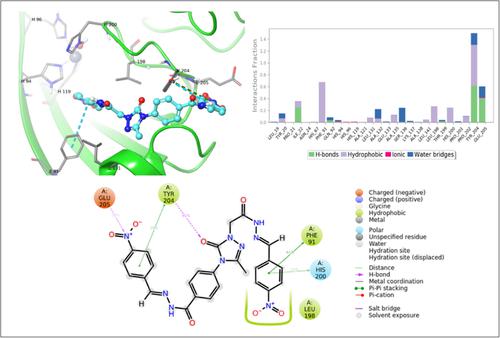
This study reports a rapid and efficient synthesis of four novel aryl Schiff base derivatives. Biological activity and molecular modeling studies were conducted to evaluate the inhibitory effects of these compounds on human carbonic anhydrases (hCA) and cholinesterases. The results indicate that the triazole‐ring‐containing compounds have strong inhibitory effects on hCA I, hCA II, acetylcholinesterase (AChE), and butyrylcholinesterase (BuChE) targets. Besides comparing the Schiff bases synthesized in our study to reference molecules, we conducted in silico investigations to examine how these compounds interact with their targets. Our studies revealed that these compounds can occupy binding sites and establish interactions with crucial residues, thus inhibiting the functions of the targets. These findings have significant implications as they can be utilized to develop more potent compounds for treating the diseases that these target proteins play crucial roles in or to obtain drug precursors with enhanced efficacy.
中文翻译:

芳基席夫碱衍生物的高效、快速和高产率合成及其对 hCA I、hCA II、AChE 和 BuChE 的体外和计算机抑制研究
本研究报告了四种新型芳基希夫碱衍生物的快速有效合成。进行了生物活性和分子模型研究,以评估这些化合物对人碳酸酐酶 (hCA) 和胆碱酯酶的抑制作用。结果表明,含三唑环的化合物对hCA I、hCA II、乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)靶标具有较强的抑制作用。除了将我们研究中合成的希夫碱与参考分子进行比较之外,我们还进行了计算机研究,以检查这些化合物如何与其靶标相互作用。我们的研究表明,这些化合物可以占据结合位点并与关键残基建立相互作用,从而抑制靶标的功能。这些发现具有重要意义,因为它们可用于开发更有效的化合物来治疗这些靶蛋白在其中发挥关键作用的疾病,或获得具有增强功效的药物前体。
更新日期:2024-04-09
中文翻译:

芳基席夫碱衍生物的高效、快速和高产率合成及其对 hCA I、hCA II、AChE 和 BuChE 的体外和计算机抑制研究
本研究报告了四种新型芳基希夫碱衍生物的快速有效合成。进行了生物活性和分子模型研究,以评估这些化合物对人碳酸酐酶 (hCA) 和胆碱酯酶的抑制作用。结果表明,含三唑环的化合物对hCA I、hCA II、乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)靶标具有较强的抑制作用。除了将我们研究中合成的希夫碱与参考分子进行比较之外,我们还进行了计算机研究,以检查这些化合物如何与其靶标相互作用。我们的研究表明,这些化合物可以占据结合位点并与关键残基建立相互作用,从而抑制靶标的功能。这些发现具有重要意义,因为它们可用于开发更有效的化合物来治疗这些靶蛋白在其中发挥关键作用的疾病,或获得具有增强功效的药物前体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号