当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Disproof of the Structures and Biosynthesis of Ergoynes, Gs-Polyyne–l-Ergothioneine Cycloadducts from Gynuella sunshinyii YC6258
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-09 , DOI: 10.1021/acs.joc.4c00243 Daiki Kawahara 1 , Kenji Kai 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-09 , DOI: 10.1021/acs.joc.4c00243 Daiki Kawahara 1 , Kenji Kai 1
Affiliation
|
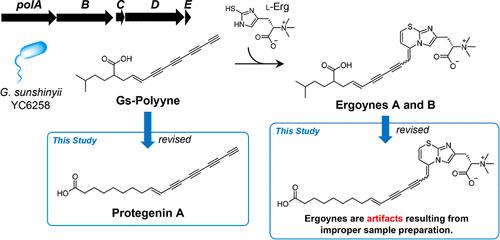
Some bacteria produce “bacterial polyynes” bearing a conjugated C≡C bond that starts with a terminal alkyne. Ergoynes A and B have been reported as sulfur-containing metabolites from Gynuella sunshinyii YC6258. These compounds were thought to be formed by cycloaddition between a bacterial polyyne (named Gs-polyyne) and l-ergothioneine. The biosynthetic gene clusters (BGCs), which may contribute to their synthesis, were present in the YC6258 genome. The biosynthetic origin of Gs-polyyne is interesting considering its rare 2-isopentyl fatty acyl skeleton. Here, the structures and biosynthesis of Gs-polyyne and ergoynes were verified by analytical, chemical, and genetic techniques. In the YC6258 extract, which was prepared considering their instability, Gs-polyyne was detected as a major LC peak, and ergoynes were not detected. The NMR data of the isolated Gs-polyyne contradicted the proposed structure and identified it as the previously reported protegenin A. The expression of Gs-polyyne BGC in Escherichia coli BL21(DE3) also yielded protegenin A. The cyclization between protegenin A and l-ergothioneine did not proceed during sample preparation; a base, such as potassium carbonate, was required. Overall, Gs-polyyne was identified as protegenin A, while ergoynes were determined to be artifacts. This cyclization may provide a derivatization to stabilize polyynes or create new chemical space.
中文翻译:

甘努菌 YC6258 中麦角素、Gs-聚炔-l-麦角硫因环导管的结构和生物合成的反证
一些细菌产生“细菌多炔”,其带有以末端炔烃开头的共轭 C=C 键。据报道,Ergoynes A 和 B 是来自Gynuella sunshinyii YC6258的含硫代谢物。这些化合物被认为是通过细菌多炔(称为Gs-多炔)和l-麦角硫因之间的环加成形成的。 YC6258 基因组中存在可能有助于其合成的生物合成基因簇 (BGC)。考虑到其罕见的 2-异戊基脂肪酰基骨架,Gs-聚炔的生物合成起源很有趣。在这里,Gs-聚炔和麦角炔的结构和生物合成通过分析、化学和遗传技术进行了验证。在考虑到其不稳定性而制备的YC6258提取物中,检测到Gs-聚炔作为主要LC峰,并且未检测到麦角炔。分离的 Gs-多炔的 NMR 数据与所提出的结构相矛盾,并将其鉴定为先前报道的 protegenin A。Gs-多炔 BGC 在大肠杆菌BL21(DE3) 中的表达也产生了 protegenin A。protegenin A 和l -之间的环化麦角硫因在样品制备过程中未进行;需要碱,例如碳酸钾。总体而言,Gs-聚炔被鉴定为 protegenin A,而麦角炔被确定为伪影。这种环化可以提供衍生作用以稳定多炔或产生新的化学空间。
更新日期:2024-04-09
中文翻译:

甘努菌 YC6258 中麦角素、Gs-聚炔-l-麦角硫因环导管的结构和生物合成的反证
一些细菌产生“细菌多炔”,其带有以末端炔烃开头的共轭 C=C 键。据报道,Ergoynes A 和 B 是来自Gynuella sunshinyii YC6258的含硫代谢物。这些化合物被认为是通过细菌多炔(称为Gs-多炔)和l-麦角硫因之间的环加成形成的。 YC6258 基因组中存在可能有助于其合成的生物合成基因簇 (BGC)。考虑到其罕见的 2-异戊基脂肪酰基骨架,Gs-聚炔的生物合成起源很有趣。在这里,Gs-聚炔和麦角炔的结构和生物合成通过分析、化学和遗传技术进行了验证。在考虑到其不稳定性而制备的YC6258提取物中,检测到Gs-聚炔作为主要LC峰,并且未检测到麦角炔。分离的 Gs-多炔的 NMR 数据与所提出的结构相矛盾,并将其鉴定为先前报道的 protegenin A。Gs-多炔 BGC 在大肠杆菌BL21(DE3) 中的表达也产生了 protegenin A。protegenin A 和l -之间的环化麦角硫因在样品制备过程中未进行;需要碱,例如碳酸钾。总体而言,Gs-聚炔被鉴定为 protegenin A,而麦角炔被确定为伪影。这种环化可以提供衍生作用以稳定多炔或产生新的化学空间。



























 京公网安备 11010802027423号
京公网安备 11010802027423号