当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Li2MP2S6: Building-Block Approach to a Family of 2D Non-van der Waals-Layered Materials and Their Water, Ammonia, and Ion Intercalation Properties
Chemistry of Materials ( IF 8.6 ) Pub Date : 2024-04-05 , DOI: 10.1021/acs.chemmater.3c02829 Santhoshkumar Sundaramoorthy 1 , Nikolay Gerasimchuk 2 , Kartik Ghosh 3 , Steven P. Kelley 4 , Amitava Choudhury 1
Chemistry of Materials ( IF 8.6 ) Pub Date : 2024-04-05 , DOI: 10.1021/acs.chemmater.3c02829 Santhoshkumar Sundaramoorthy 1 , Nikolay Gerasimchuk 2 , Kartik Ghosh 3 , Steven P. Kelley 4 , Amitava Choudhury 1
Affiliation
|
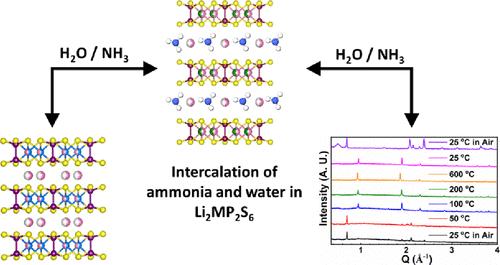
A series of non-van der Waals 2D quaternary thiophosphates with nominal composition, Li2MP2S6 (M = V, Mn, Fe, Co, Ni, and Zn), has been successfully synthesized through solid-state metathesis reactions by a building block approach as well as starting with a stoichiometric combination of elements. Li4–nxMxn+P2S6 (M = V, Fe, Ni, Co, and Zn; x = 0.5–1, n = 2 or 3) crystallize in the P3̅1m space group, while Li2MnP2S6 crystallizes in R3̅. All the compounds form a 2D layered structure through edge sharing MS6 octahedra and P2S6 units with Li atoms occupying the interlayer spaces. X-ray diffraction and thermogravimetric analyses reveal spontaneous water intercalation tendencies of these materials, leading to two distinct hydrated phases (HY-I and HY-II) when they are exposed to air for shorter and extended times, respectively. Thermodiffractograms demonstrate the reversibility of phase transformation upon deintercalation of water molecules from the interlayer regions. The crystal structure of hydrated phase I from single-crystal and synchrotron powder X-ray diffraction indicates formation of a monolayer of water with interlayer expansion. Additionally, Li4–nxMxn+P2S6 (M = V, Mn, Fe, and Ni) also display huge affinity toward NH3 intercalation in the interlayer space when subjected to a liquid or gaseous ammonia environment. The magnetic measurements on Li2MP2S6 (M = Mn and Ni) show the paramagnetic nature of the compounds down to 2 K. AC impedance spectroscopy on Li2.56Zn0.72P2S6 shows a room-temperature ionic conductivity of 2.69 × 10–3 mS/cm, which is four order higher in magnitude than Li4P2S6, while hydrated Li2.56Zn0.72P2S6 display 7-fold higher ionic conductivity (1.85 × 10–2 mS/cm) than its anhydrous counterpart. The study also reports electrochemical Li (de)intercalation in Li2FeP2S6 in a Li-ion battery with a liquid electrolyte for the first time.
中文翻译:

Li2MP2S6:二维非范德华层状材料及其水、氨和离子嵌入特性系列的构建方法
通过固相复分解反应,成功合成了一系列标称成分为 Li 2 MP 2 S 6 (M = V、Mn、Fe、Co、Ni 和 Zn)的非范德华二维季硫代磷酸盐。构建块方法以及从元素的化学计量组合开始。 Li 4– n x M x n + P 2 S 6(M = V、Fe、Ni、Co 和 Zn;x = 0.5–1,n = 2 或 3)在P 3̅1 m空间群中结晶,而 Li 2 MnP 2 S 6在R 3̅中结晶。所有化合物通过共享MS 6八面体和P 2 S 6单元形成二维层状结构,Li原子占据层间空间。 X 射线衍射和热重分析揭示了这些材料的自发水嵌入倾向,当它们暴露在空气中较短和较长时间时,分别产生两个不同的水合相(HY-I 和 HY-II)。热衍射图证明了水分子从层间区域脱嵌时相变的可逆性。单晶和同步加速器粉末 X 射线衍射的水合相 I 的晶体结构表明形成了具有层间膨胀的单层水。此外,当处于液态或气态氨环境中时,Li 4– nx M x n + P 2 S 6 (M = V、Mn、Fe和Ni)也对层间空间中的NH 3插层表现出巨大的亲和力。 Li 2 MP 2 S 6 (M = Mn 和 Ni)的磁性测量显示化合物在低至 2 K 时具有顺磁性。Li 2.56 Zn 0.72 P 2 S 6的交流阻抗谱显示室温离子电导率为 2.69 × 10 –3 mS/cm,比 Li 4 P 2 S 6高四个数量级,而水合 Li 2.56 Zn 0.72 P 2 S 6的离子电导率高出 7 倍(1.85 × 10 –2 mS/cm)比它的无水对应物。该研究还报告了 Li 2 FeP 2 S 6中的电化学 Li(脱)嵌首次在具有液体电解质的锂离子电池中。
更新日期:2024-04-05
中文翻译:

Li2MP2S6:二维非范德华层状材料及其水、氨和离子嵌入特性系列的构建方法
通过固相复分解反应,成功合成了一系列标称成分为 Li 2 MP 2 S 6 (M = V、Mn、Fe、Co、Ni 和 Zn)的非范德华二维季硫代磷酸盐。构建块方法以及从元素的化学计量组合开始。 Li 4– n x M x n + P 2 S 6(M = V、Fe、Ni、Co 和 Zn;x = 0.5–1,n = 2 或 3)在P 3̅1 m空间群中结晶,而 Li 2 MnP 2 S 6在R 3̅中结晶。所有化合物通过共享MS 6八面体和P 2 S 6单元形成二维层状结构,Li原子占据层间空间。 X 射线衍射和热重分析揭示了这些材料的自发水嵌入倾向,当它们暴露在空气中较短和较长时间时,分别产生两个不同的水合相(HY-I 和 HY-II)。热衍射图证明了水分子从层间区域脱嵌时相变的可逆性。单晶和同步加速器粉末 X 射线衍射的水合相 I 的晶体结构表明形成了具有层间膨胀的单层水。此外,当处于液态或气态氨环境中时,Li 4– nx M x n + P 2 S 6 (M = V、Mn、Fe和Ni)也对层间空间中的NH 3插层表现出巨大的亲和力。 Li 2 MP 2 S 6 (M = Mn 和 Ni)的磁性测量显示化合物在低至 2 K 时具有顺磁性。Li 2.56 Zn 0.72 P 2 S 6的交流阻抗谱显示室温离子电导率为 2.69 × 10 –3 mS/cm,比 Li 4 P 2 S 6高四个数量级,而水合 Li 2.56 Zn 0.72 P 2 S 6的离子电导率高出 7 倍(1.85 × 10 –2 mS/cm)比它的无水对应物。该研究还报告了 Li 2 FeP 2 S 6中的电化学 Li(脱)嵌首次在具有液体电解质的锂离子电池中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号