当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Prolinamides derived from 2-aminocyclohexanols as organocatalysts for asymmetric List-Lerner-Barbas aldol reactions
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2024-03-27 , DOI: 10.1016/j.tetlet.2024.155042 Tolga A. Yeşil , Taner Atalar , Mustafa Yavuz , Erkan Ertürk
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2024-03-27 , DOI: 10.1016/j.tetlet.2024.155042 Tolga A. Yeşil , Taner Atalar , Mustafa Yavuz , Erkan Ertürk
|
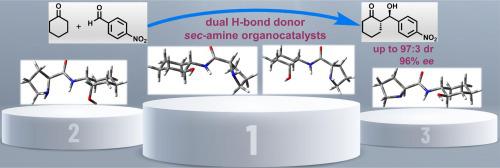
Starting from Cbz--proline and either racemic -2-aminocyclohexanol or racemic -2-aminocyclohexanol, new chiral prolinamides containing 2-aminocyclohexanols have been made available in an effective manner. They are demonstrated to be capable of catalyzing the List-Lerner-Barbas aldol reaction between ketones and aldehydes. While they generally show moderate catalytic activity, they provide diastereomeric ratio up to 97:3 () and 96% in the reaction of cyclohexanone with benzaldehyde. Computational studies reveal that the electrophile (e.g. aldehyde) is activated by two-point hydrogen bonding with the amide NH and the OH protons of the catalyst.
中文翻译:

2-氨基环己醇衍生的脯氨酰胺作为不对称 List-Lerner-Barbas 羟醛反应的有机催化剂
从Cbz-脯氨酸和外消旋-2-氨基环己醇或外消旋-2-氨基环己醇开始,已经以有效的方式制备了含有2-氨基环己醇的新型手性脯氨酰胺。它们被证明能够催化酮和醛之间的 List-Lerner-Barbas 羟醛反应。虽然它们通常表现出中等的催化活性,但在环己酮与苯甲醛的反应中,它们的非对映异构体比例高达 97:3 () 和 96%。计算研究表明,亲电子试剂(例如醛)通过与催化剂的酰胺 NH 和 OH 质子的两点氢键被激活。
更新日期:2024-03-27
中文翻译:

2-氨基环己醇衍生的脯氨酰胺作为不对称 List-Lerner-Barbas 羟醛反应的有机催化剂
从Cbz-脯氨酸和外消旋-2-氨基环己醇或外消旋-2-氨基环己醇开始,已经以有效的方式制备了含有2-氨基环己醇的新型手性脯氨酰胺。它们被证明能够催化酮和醛之间的 List-Lerner-Barbas 羟醛反应。虽然它们通常表现出中等的催化活性,但在环己酮与苯甲醛的反应中,它们的非对映异构体比例高达 97:3 () 和 96%。计算研究表明,亲电子试剂(例如醛)通过与催化剂的酰胺 NH 和 OH 质子的两点氢键被激活。



























 京公网安备 11010802027423号
京公网安备 11010802027423号