当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Helical Content Correlations and Hydration Structures of the Folding Ensemble of the B Domain of Protein A
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-04-02 , DOI: 10.1021/acs.jcim.3c01822 Ander Francisco Pereira 1 , Leandro Martínez 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-04-02 , DOI: 10.1021/acs.jcim.3c01822 Ander Francisco Pereira 1 , Leandro Martínez 1
Affiliation
|
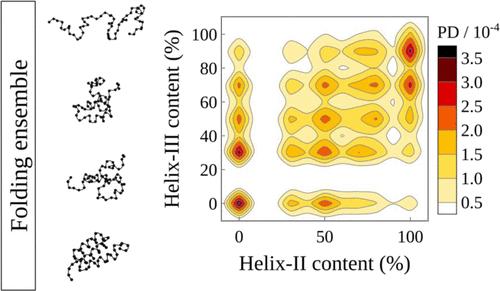
The B domain of protein A (BdpA), a small three-helix bundle, folds on a time scale of a few microseconds with heterogeneous native and unfolded states. It is widely used as a model for understanding protein folding mechanisms. In this work, we use structure-based models (SBMs) and atomistic simulations to comprehensively investigate how BdpA folding is associated with the formation of its secondary structure. The energy landscape visualization method (ELViM) was used to characterize the pathways that connect the folded and unfolded states of BdpA as well as the sets of structures displaying specific ellipticity patterns. We show that the native state conformational diversity is due mainly to the conformational variability of helix I. Helices I, II, and III occur in a weakly correlated manner, with Spearman’s rank correlation coefficients of 0.1539 (I and II), 0.1259 (I and III), and 0.2561 (II and III). These results, therefore, suggest the highest cooperativity between helices II and III. Our results allow the clustering of partially folded structures of folding of the B domain of protein A on the basis of its secondary structure, paving the way to an understanding of environmental factors in the relative stability of the basins of the folding ensemble, which are illustrated by the structural dependency of the protein hydration structures, as computed with minimum-distance distribution functions.
中文翻译:

蛋白质 A B 结构域折叠整体的螺旋含量相关性和水合结构
蛋白 A (BdpA) 的 B 结构域是一个小的三螺旋束,在几微秒的时间内折叠,具有异质的天然和未折叠状态。它被广泛用作理解蛋白质折叠机制的模型。在这项工作中,我们使用基于结构的模型(SBM)和原子模拟来全面研究 BdpA 折叠与其二级结构形成的关系。能量景观可视化方法 (ELViM) 用于表征连接 BdpA 折叠和展开状态的路径以及显示特定椭圆率模式的结构集。我们表明,天然状态构象多样性主要是由于螺旋 I 的构象变异性。螺旋 I、II 和 III 以弱相关方式出现,Spearman 等级相关系数为 0.1539(I 和 II)、0.1259(I 和 II)、0.1259(I 和 II)。 III) 和 0.2561 (II 和 III)。因此,这些结果表明螺旋 II 和 III 之间的协同性最高。我们的结果允许根据其二级结构对蛋白质 A B 结构域折叠的部分折叠结构进行聚类,为理解折叠整体盆地相对稳定性中的环境因素铺平了道路,如下所示通过蛋白质水合结构的结构依赖性,用最小距离分布函数计算。
更新日期:2024-04-02
中文翻译:

蛋白质 A B 结构域折叠整体的螺旋含量相关性和水合结构
蛋白 A (BdpA) 的 B 结构域是一个小的三螺旋束,在几微秒的时间内折叠,具有异质的天然和未折叠状态。它被广泛用作理解蛋白质折叠机制的模型。在这项工作中,我们使用基于结构的模型(SBM)和原子模拟来全面研究 BdpA 折叠与其二级结构形成的关系。能量景观可视化方法 (ELViM) 用于表征连接 BdpA 折叠和展开状态的路径以及显示特定椭圆率模式的结构集。我们表明,天然状态构象多样性主要是由于螺旋 I 的构象变异性。螺旋 I、II 和 III 以弱相关方式出现,Spearman 等级相关系数为 0.1539(I 和 II)、0.1259(I 和 II)、0.1259(I 和 II)。 III) 和 0.2561 (II 和 III)。因此,这些结果表明螺旋 II 和 III 之间的协同性最高。我们的结果允许根据其二级结构对蛋白质 A B 结构域折叠的部分折叠结构进行聚类,为理解折叠整体盆地相对稳定性中的环境因素铺平了道路,如下所示通过蛋白质水合结构的结构依赖性,用最小距离分布函数计算。



























 京公网安备 11010802027423号
京公网安备 11010802027423号