当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-Selective S-Arylation of 1-Thiosugars with Aryl Thianthrenium Salts through Copper(I)-Mediated, Photoredox- Catalyzed Reactions
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-04-04 , DOI: 10.1002/adsc.202400121 Yi-Ni Fang 1 , Qing Liang 1 , Lingling Shi 1 , Jiayang Wen 1 , Xinzhang Liu 1 , Xuerui Hong 1 , Xiaoming Zha 1 , Fei Ji 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-04-04 , DOI: 10.1002/adsc.202400121 Yi-Ni Fang 1 , Qing Liang 1 , Lingling Shi 1 , Jiayang Wen 1 , Xinzhang Liu 1 , Xuerui Hong 1 , Xiaoming Zha 1 , Fei Ji 1
Affiliation
|
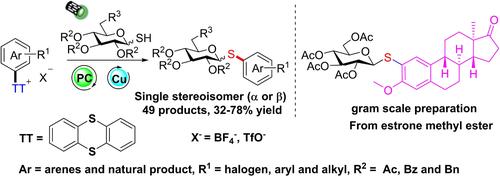
A process for the synthesis of aryl thioglycosides from the aryl thianthrenium salts and 1‐thiosugars is achieved by copper(I)‐mediated, photoredox‐catalyzed reactions. The desired products could be obtained in 32% to 78% yield after irradiation with 34 W blue light at room temperature. Various functional groups, especially including halogen groups, were well tolerated under standard reaction conditions. This strategy differs conceptually from all previous S‐glycosylations in that thiosugar functionality could be incorporated into complex natural products or drugs at a late stage site‐selectively, which has not been shown via aryl halides and aryldiazonium salts.
中文翻译:

通过铜 (I) 介导的光氧化还原催化反应对 1-硫代糖与芳基噻烯盐进行位点选择性 S 芳基化
通过铜(I)介导的光氧化还原催化反应,实现了从芳基噻啉鎓盐和 1-硫代糖合成芳基硫代糖苷的方法。室温下34 W蓝光照射后,可以得到目标产物,收率32%至78%。各种官能团,尤其是卤素基团,在标准反应条件下具有良好的耐受性。该策略在概念上不同于之前所有的 S-糖基化,因为硫代糖功能可以在后期选择性地结合到复杂的天然产物或药物中,而芳基卤化物和芳基重氮盐尚未显示出这一点。
更新日期:2024-04-04
中文翻译:

通过铜 (I) 介导的光氧化还原催化反应对 1-硫代糖与芳基噻烯盐进行位点选择性 S 芳基化
通过铜(I)介导的光氧化还原催化反应,实现了从芳基噻啉鎓盐和 1-硫代糖合成芳基硫代糖苷的方法。室温下34 W蓝光照射后,可以得到目标产物,收率32%至78%。各种官能团,尤其是卤素基团,在标准反应条件下具有良好的耐受性。该策略在概念上不同于之前所有的 S-糖基化,因为硫代糖功能可以在后期选择性地结合到复杂的天然产物或药物中,而芳基卤化物和芳基重氮盐尚未显示出这一点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号