当前位置:
X-MOL 学术
›
Food Chem. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fasting potentiates diclofenac-induced liver injury via inductions of oxidative/endoplasmic reticulum stresses and apoptosis, and inhibition of autophagy by depleting hepatic glutathione in mice
Food and Chemical Toxicology ( IF 4.3 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.fct.2024.114624 Doyoung Kwon , Hyeji Seo , Sou Hyun Kim , Ki Wung Chung , Jaewon Lee , Young-Suk Jung
Food and Chemical Toxicology ( IF 4.3 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.fct.2024.114624 Doyoung Kwon , Hyeji Seo , Sou Hyun Kim , Ki Wung Chung , Jaewon Lee , Young-Suk Jung
|
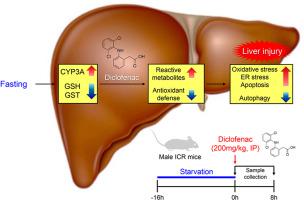
Diclofenac, a widely used non-steroidal anti-inflammatory drug, can cause liver damage via its metabolic activation by hepatic CYP450s and UGT2B7. Fasting can affect drug-induced liver injury by modulating the hepatic metabolism, but its influence on diclofenac hepatotoxicity is unknown. Thus, we investigated diclofenac-induced liver damage after fasting in mice, and the cellular events were examined. Male ICR mice fasted for 16 h showed the elevation of CYP3A11, but the decreases of UGT2B7, glutathione (GSH), and GSH S-transferase-μ/-π levels in the livers. Diclofenac (200 mg/kg) injection into the mice after 16-h fasting caused more significant liver damage compared to that in the diclofenac-treated fed mice, as shown by the higher serum ALT and AST activities. Diclofenac-promoted hepatic oxidative stress (oxidized proteins, 4-hydroxynonenal, and malondialdehyde), endoplasmic reticulum (ER) stress (BiP, ATF6, and CHOP), and apoptosis (cleaved caspase-3 and cleaved PARP) were enhanced by fasting. Autophagic degradation was inhibited in the diclofenac-treated fasting mice compared to that of the corresponding fed mice. The results suggest that fasting can make the liver more susceptible to diclofenac toxicity by lowering GSH-mediated detoxification; increased oxidative/ER stresses and apoptosis and suppressed autophagic degradation may be the cellular mechanisms of the aggravated diclofenac hepatotoxicity under fasting conditions.
中文翻译:

禁食可通过诱导氧化/内质网应激和细胞凋亡,并通过消耗肝脏谷胱甘肽来抑制自噬,从而增强双氯芬酸诱导的肝损伤
双氯芬酸是一种广泛使用的非甾体类抗炎药,可通过肝脏 CYP450 和 UGT2B7 的代谢激活引起肝损伤。禁食可通过调节肝脏代谢来影响药物性肝损伤,但其对双氯芬酸肝毒性的影响尚不清楚。因此,我们研究了双氯芬酸在小鼠禁食后引起的肝损伤,并检查了细胞事件。禁食16小时的雄性ICR小鼠显示肝脏中CYP3A11升高,但UGT2B7、谷胱甘肽(GSH)和GSH S-转移酶-μ/-π水平降低。与双氯芬酸处理的喂养小鼠相比,禁食 16 小时后向小鼠注射双氯芬酸(200 mg/kg)引起更显着的肝损伤,如血清 ALT 和 AST 活性较高所示。禁食可增强双氯芬酸促进的肝脏氧化应激(氧化蛋白、4-羟基壬烯醛和丙二醛)、内质网 (ER) 应激(BiP、ATF6 和 CHOP)和细胞凋亡(裂解的 caspase-3 和裂解的 PARP)。与相应进食的小鼠相比,双氯芬酸处理的禁食小鼠的自噬降解受到抑制。结果表明,禁食可以通过降低 GSH 介导的解毒作用,使肝脏更容易受到双氯芬酸毒性的影响;氧化/内质网应激和细胞凋亡增加以及自噬降解受到抑制可能是空腹条件下双氯芬酸肝毒性加重的细胞机制。
更新日期:2024-03-29
中文翻译:

禁食可通过诱导氧化/内质网应激和细胞凋亡,并通过消耗肝脏谷胱甘肽来抑制自噬,从而增强双氯芬酸诱导的肝损伤
双氯芬酸是一种广泛使用的非甾体类抗炎药,可通过肝脏 CYP450 和 UGT2B7 的代谢激活引起肝损伤。禁食可通过调节肝脏代谢来影响药物性肝损伤,但其对双氯芬酸肝毒性的影响尚不清楚。因此,我们研究了双氯芬酸在小鼠禁食后引起的肝损伤,并检查了细胞事件。禁食16小时的雄性ICR小鼠显示肝脏中CYP3A11升高,但UGT2B7、谷胱甘肽(GSH)和GSH S-转移酶-μ/-π水平降低。与双氯芬酸处理的喂养小鼠相比,禁食 16 小时后向小鼠注射双氯芬酸(200 mg/kg)引起更显着的肝损伤,如血清 ALT 和 AST 活性较高所示。禁食可增强双氯芬酸促进的肝脏氧化应激(氧化蛋白、4-羟基壬烯醛和丙二醛)、内质网 (ER) 应激(BiP、ATF6 和 CHOP)和细胞凋亡(裂解的 caspase-3 和裂解的 PARP)。与相应进食的小鼠相比,双氯芬酸处理的禁食小鼠的自噬降解受到抑制。结果表明,禁食可以通过降低 GSH 介导的解毒作用,使肝脏更容易受到双氯芬酸毒性的影响;氧化/内质网应激和细胞凋亡增加以及自噬降解受到抑制可能是空腹条件下双氯芬酸肝毒性加重的细胞机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号