当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and characterization of ethyl 3‐benzoyl‐7‐morpholinoindolizine‐1‐carboxylate as anti‐tubercular agents: In silico screening for possible target identification
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2024-04-04 , DOI: 10.1111/cbdd.14512 Priya Tiwari 1 , Gayakvad Sunitaben Mangubhai 1 , Saqib Kidwai 2 , Ramandeep Singh 2 , Sandeep Chandrashekharappa 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2024-04-04 , DOI: 10.1111/cbdd.14512 Priya Tiwari 1 , Gayakvad Sunitaben Mangubhai 1 , Saqib Kidwai 2 , Ramandeep Singh 2 , Sandeep Chandrashekharappa 1
Affiliation
|
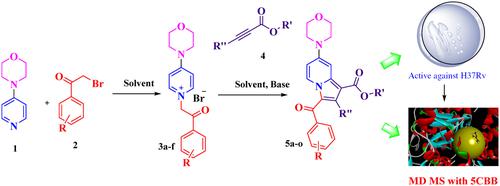
A thorough search for the development of innovative drugs to treat tuberculosis, especially considering the urgent need to address developing drug resistance, we report here a synthetic series of ethyl 3‐benzoyl‐7‐morpholinoindolizine‐1‐carboxylate analogues (5a‐o) as potent anti‐tubercular agents. These morpholino‐indolizines were synthesized by reacting 4‐morpholino pyridinium salts, with various electron‐deficient acetylenes to afford the ethyl 3‐benzoyl‐7‐morpholinoindolizine‐1‐carboxylate analogues (5a‐o). All synthesized intermediate and final compounds are characterized by spectroscopic methods such as 1 H NMR, 13 C NMR and HRMS and further examined for their anti‐tubercular activity against the M. tuberculosis H37Rv strain (ATCC 27294—American type cell culture). All the compounds screened for anti‐tubercular activity in the range of 6.25–50 μM against the H37Rv strain of Mycobacterium tuberculosis . Compound 5g showed prominent activity with MIC99 2.55 μg/mL whereas compounds 5d and 5j showed activity with MIC99 18.91 μg/mL and 25.07 μg/mL, respectively. In silico analysis of these compounds revealed drug‐likeness. Additionally, the molecular target identification for Malate synthase (PDB 5CBB) is attained by computational approach. The compound 5g with a MIC99 value of 2.55 μg/mL against M. tuberculosis H37Rv emerged as the most promising anti‐TB drug and in silico investigations suggest Malate synthase (5CBB) might be the compound's possible target.
中文翻译:

抗结核药物 3-苯甲酰基-7-吗啉代吲嗪-1-甲酸乙酯的设计、合成和表征:通过计算机筛选可能的靶点识别
为了彻底探索治疗结核病的创新药物的开发,特别是考虑到迫切需要解决耐药性的产生,我们在此报告了一系列合成的 3-苯甲酰基-7-吗啉代吲嗪-1-羧酸酯类似物 (5a-o) 如下强效抗结核药。这些吗啉代中氮茚是通过 4-吗啉代吡啶鎓盐与各种缺电子乙炔反应合成的,得到 3-苯甲酰基-7-吗啉代中氮茚-1-羧酸乙酯类似物 (5a-o)。所有合成的中间体和最终化合物均通过光谱方法进行表征,例如1 核磁共振氢谱,13 13 C NMR 和 HRMS 并进一步检查了它们的抗结核活性结核分枝杆菌 H37Rv 株(ATCC 27294—美国型细胞培养物)。所有化合物均在 6.25-50 μM 范围内筛选出针对 H37Rv 菌株的抗结核活性。结核分枝杆菌 。化合物 5g 显示出显着的 MIC 活性99 2.55 μg/mL,而化合物 5d 和 5j 显示出 MIC 活性99 分别为 18.91 μg/mL 和 25.07 μg/mL。这些化合物的计算机分析揭示了药物相似性。此外,通过计算方法实现了苹果酸合酶(PDB 5CBB)的分子靶点鉴定。化合物 5g,MIC99 2.55 μg/mL 的值结核分枝杆菌 H37Rv 成为最有前途的抗结核药物,计算机研究表明苹果酸合酶 (5CBB) 可能是该化合物的可能靶点。
更新日期:2024-04-04
中文翻译:

抗结核药物 3-苯甲酰基-7-吗啉代吲嗪-1-甲酸乙酯的设计、合成和表征:通过计算机筛选可能的靶点识别
为了彻底探索治疗结核病的创新药物的开发,特别是考虑到迫切需要解决耐药性的产生,我们在此报告了一系列合成的 3-苯甲酰基-7-吗啉代吲嗪-1-羧酸酯类似物 (5a-o) 如下强效抗结核药。这些吗啉代中氮茚是通过 4-吗啉代吡啶鎓盐与各种缺电子乙炔反应合成的,得到 3-苯甲酰基-7-吗啉代中氮茚-1-羧酸乙酯类似物 (5a-o)。所有合成的中间体和最终化合物均通过光谱方法进行表征,例如



























 京公网安备 11010802027423号
京公网安备 11010802027423号