当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The structural evolution of CL-20-based energetic host–guest solvates at decomposition temperature according to the perceptions of THz spectroscopy
CrystEngComm ( IF 3.1 ) Pub Date : 2024-04-03 , DOI: 10.1039/d3ce01310c Lu Shi 1 , XiaoHui Duan 2 , Hongzhen Li 3
CrystEngComm ( IF 3.1 ) Pub Date : 2024-04-03 , DOI: 10.1039/d3ce01310c Lu Shi 1 , XiaoHui Duan 2 , Hongzhen Li 3
Affiliation
|
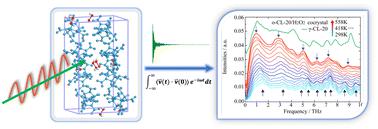
Because of their unique physicochemical integration mode, energetic host–guest solvates provide a balanced method to expand CL-20 high explosives. CL-20/hydrogen peroxide (H2O2) energetic solvates maintain their detonation performance by combining H2O2 molecules into a lattice akin to hydrate CL-20/H2O solvate (α-CL-20). Herein, using periodic density functional theory (DFT) calculation and molecular dynamics simulation, the structural evolution and intermolecular interaction mechanism of orthorhombic CL-20/H2O2 (o-CL-20/H2O2), monoclinic CL-20/H2O2 (m-CL-20/H2O2) and CL-20/H2O solvates were monitored by the vibrational spectrum and vibrational density of states (VDOS) in the THz region when the temperature was increased from 298 K to 558 K. There was greater similarity in the vibrational characteristics of THz spectra for o-CL-20/H2O2 and CL-20/H2O solvates that was supported by the thermal expansion anisotropy of lattice parameters and that of difference for the m-CL-20/H2O2 solvate. With increased temperature, spectral signals in the power spectrum of VDOS reflected the conformational evolution of CL-20 molecules in o-CL-20/H2O2 solvate and structural insensitivity of m-CL-20/H2O2 solvate. THz peaks of CL-20/H2O revealed the weakening of intermolecular interactions of CL-20 and H2O molecules, which were in agreement with the fact that H2O molecules more easily escape from host–guest solvates, whereas the H2O2 molecule was restricted in a cage of CL-20 molecules for the m-CL-20/H2O2 solvate. RDF and hydrogen bond (H-bond) interaction analyses were performed to further clarify stronger intermolecular H-bond interactions in the o-CL-20/H2O2 solvate as compared to that of the other two combined with H2O2 or H2O solvates. This finding introduces new insights into clarifying the formation mechanism of hydroxide with CL-20-based energetic host–guest solvates.
中文翻译:

根据太赫兹光谱的感知,基于 CL-20 的高能主客体溶剂化物在分解温度下的结构演化
由于其独特的物理化学整合模式,高能主客体溶剂化物提供了一种平衡的方法来膨胀CL-20高能炸药。 CL-20/过氧化氢 (H 2 O 2 ) 高能溶剂化物通过将 H 2 O 2分子结合成类似于水合 CL-20/H 2 O 溶剂化物 (α-CL-20) 的晶格来保持其爆炸性能。本文利用周期密度泛函理论(DFT)计算和分子动力学模拟,研究了斜方晶系CL-20/H 2 O 2(o-CL-20/H 2 O 2)、单斜晶系CL-20的结构演化和分子间相互作用机制。当温度从 298 K 至 558 K。 o-CL-20/H 2 O 2和 CL-20/H 2 O 溶剂化物的太赫兹光谱振动特征具有更大的相似性,这得到了晶格参数的热膨胀各向异性的支持,并且m-CL-20/H 2 O 2溶剂化物的差异。随着温度升高,VDOS功率谱中的光谱信号反映了o-CL-20/H 2 O 2溶剂化物中CL-20分子的构象演化和m-CL-20/H 2 O 2溶剂化物的结构不敏感性。 CL-20/H 2 O的太赫兹峰揭示了CL-20和H 2 O分子间相互作用的减弱,这与H 2 O分子更容易从主客体溶剂化物中逃逸的事实相一致,而H 2 O 2分子被限制在m-CL-20/H 2 O 2溶剂化物的CL-20分子笼中。进行 RDF 和氢键 (H-bond) 相互作用分析,以进一步阐明 o-CL-20/H 2 O 2溶剂化物中的分子间氢键相互作用比其他两种与 H 2 O 2或H 2 O 2组合的溶剂化物更强。 H 2 O溶剂化物。这一发现为阐明基于 CL-20 的高能主客体溶剂化物形成氢氧化物的机制提供了新的见解。
更新日期:2024-04-03
中文翻译:

根据太赫兹光谱的感知,基于 CL-20 的高能主客体溶剂化物在分解温度下的结构演化
由于其独特的物理化学整合模式,高能主客体溶剂化物提供了一种平衡的方法来膨胀CL-20高能炸药。 CL-20/过氧化氢 (H 2 O 2 ) 高能溶剂化物通过将 H 2 O 2分子结合成类似于水合 CL-20/H 2 O 溶剂化物 (α-CL-20) 的晶格来保持其爆炸性能。本文利用周期密度泛函理论(DFT)计算和分子动力学模拟,研究了斜方晶系CL-20/H 2 O 2(o-CL-20/H 2 O 2)、单斜晶系CL-20的结构演化和分子间相互作用机制。当温度从 298 K 至 558 K。 o-CL-20/H 2 O 2和 CL-20/H 2 O 溶剂化物的太赫兹光谱振动特征具有更大的相似性,这得到了晶格参数的热膨胀各向异性的支持,并且m-CL-20/H 2 O 2溶剂化物的差异。随着温度升高,VDOS功率谱中的光谱信号反映了o-CL-20/H 2 O 2溶剂化物中CL-20分子的构象演化和m-CL-20/H 2 O 2溶剂化物的结构不敏感性。 CL-20/H 2 O的太赫兹峰揭示了CL-20和H 2 O分子间相互作用的减弱,这与H 2 O分子更容易从主客体溶剂化物中逃逸的事实相一致,而H 2 O 2分子被限制在m-CL-20/H 2 O 2溶剂化物的CL-20分子笼中。进行 RDF 和氢键 (H-bond) 相互作用分析,以进一步阐明 o-CL-20/H 2 O 2溶剂化物中的分子间氢键相互作用比其他两种与 H 2 O 2或H 2 O 2组合的溶剂化物更强。 H 2 O溶剂化物。这一发现为阐明基于 CL-20 的高能主客体溶剂化物形成氢氧化物的机制提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号