Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
TfOH-Catalyzed Facile Access for One-Pot Synthesis of β-Acylamino Ketones by Avoiding the Usage of Acetyl Chloride
Synlett ( IF 2 ) Pub Date : 2024-03-28 , DOI: 10.1055/s-0042-1751579 Sachin D. Kharat , Prasad B. Rupnavar 1 , Bapurao D. Rupanawar 2 , Mahadev P. Shinde , Keshav S. Pakhare 3 , Ayesha A. Khan 4
中文翻译:

TfOH 催化避免使用乙酰氯,一锅法轻松合成 β-酰氨基酮
更新日期:2024-03-29
Synlett ( IF 2 ) Pub Date : 2024-03-28 , DOI: 10.1055/s-0042-1751579 Sachin D. Kharat , Prasad B. Rupnavar 1 , Bapurao D. Rupanawar 2 , Mahadev P. Shinde , Keshav S. Pakhare 3 , Ayesha A. Khan 4
Affiliation
|
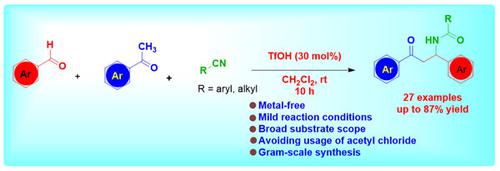
We have developed a TfOH-catalyzed, highly efficient protocol for the synthesis of biologically active β-acylamino ketones from aldehyde, ketone, and nitrile by avoiding the use of acetyl chloride. The reaction proceeds through a sequential aldol reaction followed by a nucleophilic attack of nitrile and hydrolysis of nitrile in one pot. The attractive features of this tandem process are mild reaction conditions, high atom economy, broad substrate scope with 51–87% yield, gram-scale reaction, and ease of operation.
中文翻译:

TfOH 催化避免使用乙酰氯,一锅法轻松合成 β-酰氨基酮
我们开发了一种 TfOH 催化的高效方案,通过避免使用乙酰氯,从醛、酮和腈合成具有生物活性的 β-酰氨基酮。该反应通过连续的醇醛缩合反应,然后在一锅中进行腈的亲核攻击和腈的水解。这种串联工艺的吸引人的特点是反应条件温和、原子经济性高、底物范围广、产率51-87%、克级反应和易于操作。



























 京公网安备 11010802027423号
京公网安备 11010802027423号