当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of Trisubstituted N-Phenylpyrazole Containing Diamides with Improved Insecticidal Activity
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.jafc.3c08759 Jinzhou Ren 1, 2, 3 , Xia Ji 1, 2, 3 , Jin Zhang 1, 2, 3 , Zhenwu Yu 1, 2, 3 , Xinyuan Wang 1, 2, 3 , Lixia Xiong 1, 2, 3 , Na Yang 1, 2, 3 , Liangfu Tang 1, 2, 3 , Zhengming Li 1, 2, 3 , Zhijin Fan 1, 2, 3
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.jafc.3c08759 Jinzhou Ren 1, 2, 3 , Xia Ji 1, 2, 3 , Jin Zhang 1, 2, 3 , Zhenwu Yu 1, 2, 3 , Xinyuan Wang 1, 2, 3 , Lixia Xiong 1, 2, 3 , Na Yang 1, 2, 3 , Liangfu Tang 1, 2, 3 , Zhengming Li 1, 2, 3 , Zhijin Fan 1, 2, 3
Affiliation
|
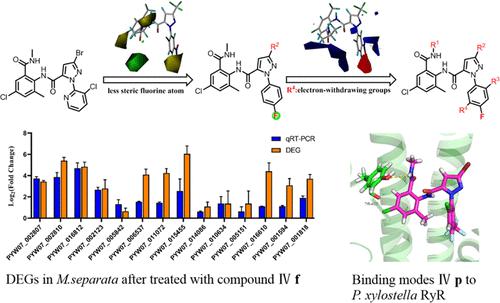
To increase the structural diversity of insecticides and meet the needs of effective integrated insect management, the structure of chlorantraniliprole was modified based on a previously established three-dimensional quantitative structure–activity relationship (3D-QSAR) model. The pyridinyl moiety in the structure of chlorantraniliprole was replaced with a 4-fluorophenyl group. Further modifications of this 4-fluorophenyl group by introducing a halogen atom at position 2 and an electron-withdrawing group (e.g., iodine, cyano, and trifluoromethyl) at position 5 led to 34 compounds with good insecticidal efficacy against Mythimna separata, Plutella xylostella, and Spodoptera frugiperda. Among them, compound IV f against M. separata showed potency comparable to that of chlorantraniliprole. IV p against P. xylostella displayed a 4.5 times higher potency than chlorantraniliprole. In addition, IV d and chlorantraniliprole exhibited comparable potencies against S. frugiperda. Transcriptome analysis showed that the molecular target of compound IV f is the ryanodine receptor. Molecular docking was further performed to verify the mode of action and insecticidal activity against resistant P. xylostella.
中文翻译:

具有改进的杀虫活性的含三取代 N-苯基吡唑二酰胺的发现
为了增加杀虫剂的结构多样性并满足有效的昆虫综合管理的需要,基于先前建立的三维定量构效关系(3D-QSAR)模型对氯虫苯甲酰胺进行了结构修饰。氯虫苯甲酰胺结构中的吡啶基部分被4-氟苯基取代。通过在2位引入卤素原子和在5位引入吸电子基团(例如碘、氰基和三氟甲基)对该4-氟苯基进行进一步修饰,得到34种对粘虫、小菜蛾、和草地贪夜蛾。其中,化合物IV f对M. separata显示出与氯虫苯甲酰胺相当的效力。IV p对抗小菜蛾的效力比氯虫苯甲酰胺高 4.5 倍。此外,IV d和氯虫苯甲酰胺对草地贪夜蛾表现出相当的效力。转录组分析表明化合物IV f的分子靶点是兰尼定受体。进一步进行分子对接,验证其对抗性小菜蛾的作用方式和杀虫活性。
更新日期:2024-03-28
中文翻译:

具有改进的杀虫活性的含三取代 N-苯基吡唑二酰胺的发现
为了增加杀虫剂的结构多样性并满足有效的昆虫综合管理的需要,基于先前建立的三维定量构效关系(3D-QSAR)模型对氯虫苯甲酰胺进行了结构修饰。氯虫苯甲酰胺结构中的吡啶基部分被4-氟苯基取代。通过在2位引入卤素原子和在5位引入吸电子基团(例如碘、氰基和三氟甲基)对该4-氟苯基进行进一步修饰,得到34种对粘虫、小菜蛾、和草地贪夜蛾。其中,化合物IV f对M. separata显示出与氯虫苯甲酰胺相当的效力。IV p对抗小菜蛾的效力比氯虫苯甲酰胺高 4.5 倍。此外,IV d和氯虫苯甲酰胺对草地贪夜蛾表现出相当的效力。转录组分析表明化合物IV f的分子靶点是兰尼定受体。进一步进行分子对接,验证其对抗性小菜蛾的作用方式和杀虫活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号