当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Electrode–Electrolyte Interface of Sodium/Glyme-Based Battery Electrolytes
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2024-03-27 , DOI: 10.1021/acs.jpcc.3c08083 Dodangodage Ishara Senadheera 1 , Orlando Carrillo-Bohorquez 1 , Ernest O. Nachaki 1 , Ryan Jorn 2 , Daniel G. Kuroda 1 , Revati Kumar 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2024-03-27 , DOI: 10.1021/acs.jpcc.3c08083 Dodangodage Ishara Senadheera 1 , Orlando Carrillo-Bohorquez 1 , Ernest O. Nachaki 1 , Ryan Jorn 2 , Daniel G. Kuroda 1 , Revati Kumar 1
Affiliation
|
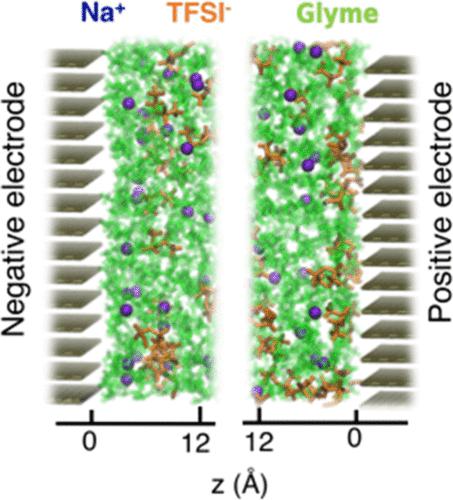
Sodium-ion batteries (NIBs) are promising systems for large-scale energy storage solutions; yet, further enhancements are required for their commercial viability. Improving the electrochemical performance of NIBs goes beyond the chemical description of the electrolyte and electrode materials as it requires a comprehensive understanding of the underlying mechanisms that govern the interface between electrodes and electrolytes. In particular, the decomposition reactions occurring at these interfaces lead to the formation of surface films. Previous work has revealed that the solvation structure of cations in the electrolyte has a significant influence on the formation and properties of these surface films. Here, an experimentally validated molecular dynamics study is performed on a 1 M NaTFSI salt in glymes of different lengths placed between two graphite electrodes having a constant bias potential. The focus of this study is on describing the solvation environment around the sodium ions at the electrode–electrolyte interface as a function of glyme chain length and applied potential. The results of the study show that the diglyme/TFSI system presents features at the interface that significantly differ from those of the triglyme/TFSI and tetraglyme/TFSI systems. These computational predictions are successfully corroborated by the experimentally measured capacitance of these systems. In addition, the dominant solvation structures at the interface explain the electrochemical stability of the system as they are consistent with cyclic voltammetry characterization.
中文翻译:

探测钠/甘醇二甲醚电池电解质的电极-电解质界面
钠离子电池(NIB)是用于大规模储能解决方案的有前景的系统;然而,其商业可行性还需要进一步增强。提高NIB的电化学性能超出了电解质和电极材料的化学描述,因为它需要全面了解控制电极和电解质之间界面的基本机制。特别是,在这些界面处发生的分解反应导致表面膜的形成。先前的工作表明,电解质中阳离子的溶剂化结构对这些表面膜的形成和性能具有显着影响。在这里,对放置在具有恒定偏压电位的两个石墨电极之间的不同长度的甘醇二甲醚中的 1 M NaTFSI 盐进行了经过实验验证的分子动力学研究。本研究的重点是描述电极-电解质界面上钠离子周围的溶剂化环境作为甘醇二甲醚链长度和施加电势的函数。研究结果表明,二甘醇二甲醚/TFSI 系统在界面处呈现出与三甘醇二甲醚/TFSI 和四甘醇二甲醚/TFSI 系统显着不同的特征。这些计算预测已被这些系统的实验测量电容成功证实。此外,界面处的主要溶剂化结构解释了系统的电化学稳定性,因为它们与循环伏安法表征一致。
更新日期:2024-03-27
中文翻译:

探测钠/甘醇二甲醚电池电解质的电极-电解质界面
钠离子电池(NIB)是用于大规模储能解决方案的有前景的系统;然而,其商业可行性还需要进一步增强。提高NIB的电化学性能超出了电解质和电极材料的化学描述,因为它需要全面了解控制电极和电解质之间界面的基本机制。特别是,在这些界面处发生的分解反应导致表面膜的形成。先前的工作表明,电解质中阳离子的溶剂化结构对这些表面膜的形成和性能具有显着影响。在这里,对放置在具有恒定偏压电位的两个石墨电极之间的不同长度的甘醇二甲醚中的 1 M NaTFSI 盐进行了经过实验验证的分子动力学研究。本研究的重点是描述电极-电解质界面上钠离子周围的溶剂化环境作为甘醇二甲醚链长度和施加电势的函数。研究结果表明,二甘醇二甲醚/TFSI 系统在界面处呈现出与三甘醇二甲醚/TFSI 和四甘醇二甲醚/TFSI 系统显着不同的特征。这些计算预测已被这些系统的实验测量电容成功证实。此外,界面处的主要溶剂化结构解释了系统的电化学稳定性,因为它们与循环伏安法表征一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号