当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-catalysed electrophilic carboamination of terminal alkynes with benzyne looked at through the computational lens
Dalton Transactions ( IF 4 ) Pub Date : 2024-03-27 , DOI: 10.1039/d3dt04301k Sven Tobisch 1
Dalton Transactions ( IF 4 ) Pub Date : 2024-03-27 , DOI: 10.1039/d3dt04301k Sven Tobisch 1
Affiliation
|
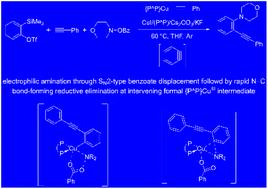
A detailed computational mechanistic study of the copper-catalysed three-component-type electrophilic carboamination of terminal alkynes with benzyne and an archetypal O-benzoylhydroxylamine electrophile is presented. Probing various plausible pathways for relevant elementary steps and scrutinising performance degradation pathways, with the aid of a reliable computational protocol applied to a realistic catalyst model combined with kinetic analysis, identified the pathways preferably traversed in productive catalysis. It entails rapid alkynylcupration of in situ generated benzyne to deliver the arylcopper nucleophile that undergoes amination with the O-benzoylhydroxylamine electrophile to afford copper benzoate. Umpolung-enabled electrophilic amination favours a multistep SN2-type oxidative addition/N–C bond-forming reductive elimination sequence involving a short-lived formal {P^P}CuIII carboxylate amido aryl intermediate. SN2-type displacement of the benzoate leaving group at the arylcopper nucleophile, which represents the catalyst resting state, is predicted to be the turnover limiting step. Alkynolysis transforms copper benzoate back to catalytically competent alkynylcopper. The computational probe of a wider range of substrates reveals that only severely ring-strained C6-arynes, C6-cycloalkynes and electron-deficient cyclopropenes featuring a highly reactive C≡C linkage could replace benzyne. Moreover, strict control of stationary benzyne concentration is indispensable for electrophilic carboamination to ever become achievable.
中文翻译:

通过计算透镜观察铜催化末端炔与苯的亲电碳胺化
提出了铜催化的末端炔烃与苯炔和典型的O-苯甲酰羟胺亲电子试剂的三组分型亲电碳胺化反应的详细计算机理研究。借助应用于现实催化剂模型的可靠计算协议并结合动力学分析,探索相关基本步骤的各种可能途径并仔细检查性能退化途径,确定了生产催化中优选经过的途径。它需要对原位产生的苯进行快速炔基化,以提供芳基铜亲核试剂,该亲核试剂与O-苯甲酰羟胺亲电试剂进行胺化,得到苯甲酸铜。 Umpolung 亲电胺化有利于多步 S N 2 型氧化加成/N-C 键形成还原消除序列,涉及短寿命的形式 {P^P}Cu III羧酸酰胺芳基中间体。芳基铜亲核体上苯甲酸酯离去基团的S N 2 型置换(代表催化剂静止状态)预计是周转限制步骤。炔解将苯甲酸铜转化回具有催化能力的炔基铜。对更广泛底物的计算探测表明,只有具有高反应性C≡C键的严重环应变C 6 -芳炔、C 6 -环炔和缺电子环丙烯才能取代苯炔。此外,严格控制固定苯炔浓度对于实现亲电碳胺化是必不可少的。
更新日期:2024-03-27
中文翻译:

通过计算透镜观察铜催化末端炔与苯的亲电碳胺化
提出了铜催化的末端炔烃与苯炔和典型的O-苯甲酰羟胺亲电子试剂的三组分型亲电碳胺化反应的详细计算机理研究。借助应用于现实催化剂模型的可靠计算协议并结合动力学分析,探索相关基本步骤的各种可能途径并仔细检查性能退化途径,确定了生产催化中优选经过的途径。它需要对原位产生的苯进行快速炔基化,以提供芳基铜亲核试剂,该亲核试剂与O-苯甲酰羟胺亲电试剂进行胺化,得到苯甲酸铜。 Umpolung 亲电胺化有利于多步 S N 2 型氧化加成/N-C 键形成还原消除序列,涉及短寿命的形式 {P^P}Cu III羧酸酰胺芳基中间体。芳基铜亲核体上苯甲酸酯离去基团的S N 2 型置换(代表催化剂静止状态)预计是周转限制步骤。炔解将苯甲酸铜转化回具有催化能力的炔基铜。对更广泛底物的计算探测表明,只有具有高反应性C≡C键的严重环应变C 6 -芳炔、C 6 -环炔和缺电子环丙烯才能取代苯炔。此外,严格控制固定苯炔浓度对于实现亲电碳胺化是必不可少的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号