当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Decamethyl-pillar[5]arene: Synthesis and Recognition Properties
Journal of Chemical Education ( IF 3 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jchemed.3c00752 Daniele Crisafulli 1 , Giulia Savoca 1 , Francesca Mancuso 1 , Martina Mazzaferro 1, 2 , Marco Milone 1 , Ilenia Pisagatti 1 , Anna Notti 1 , Melchiorre F. Parisi 1 , Giuseppe Gattuso 1
Journal of Chemical Education ( IF 3 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jchemed.3c00752 Daniele Crisafulli 1 , Giulia Savoca 1 , Francesca Mancuso 1 , Martina Mazzaferro 1, 2 , Marco Milone 1 , Ilenia Pisagatti 1 , Anna Notti 1 , Melchiorre F. Parisi 1 , Giuseppe Gattuso 1
Affiliation
|
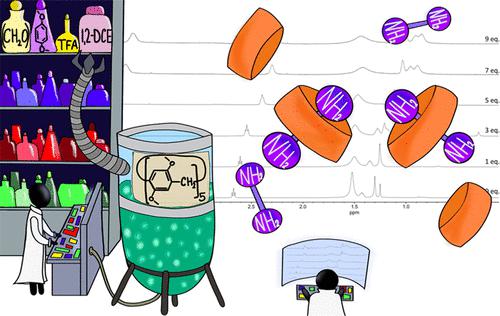
In this two-session experiment for an organic chemistry lab, students prepare a macrocyclic host compound─namely, a pillar[5]arene─by means of a templated 1,4-dimethoxybenzene/formaldehyde cyclo-oligomerization (session 1), and then, they explore its molecular recognition properties toward the 1,8-diaminooctane guest with the aid of 1H NMR spectroscopy (session 2). The acid-catalyzed synthesis of decamethyl-pillar[5]arene provides, in good yield, a product sufficiently pure to be directly used in the subsequent NMR titration experiment. The two-session experiment targets advanced chemistry students, whereas the 4 h synthesis of the macrocycle (session 1) may be included in an introductory organic chemistry laboratory course. This experiment links concepts spanning from classical SEAr reactions to macrocycle synthesis down to host–guest chemistry, NMR spectroscopy, and data treatment, providing a useful connection between organic chemistry synthesis and supramolecular chemistry.
中文翻译:

十甲基柱[5]芳烃:合成及识别性质
在这个有机化学实验室的两节实验中,学生通过模板化 1,4-二甲氧基苯/甲醛环低聚制备大环主体化合物(即柱[5]芳烃)(第 1 节),然后,他们借助1 H NMR 光谱探索了其对 1,8-二氨基辛烷客体的分子识别特性(第 2 部分)。十甲基柱[5]芳烃的酸催化合成以良好的收率提供了足够纯的产物,可直接用于后续的 NMR 滴定实验。两节实验针对的是高级化学学生,而 4 小时的大环合成(第 1 节)可能包含在介绍性有机化学实验室课程中。该实验将经典 S E Ar 反应、大环合成、主客体化学、核磁共振波谱和数据处理等概念联系起来,在有机化学合成和超分子化学之间提供了有用的联系。
更新日期:2024-03-27
中文翻译:

十甲基柱[5]芳烃:合成及识别性质
在这个有机化学实验室的两节实验中,学生通过模板化 1,4-二甲氧基苯/甲醛环低聚制备大环主体化合物(即柱[5]芳烃)(第 1 节),然后,他们借助1 H NMR 光谱探索了其对 1,8-二氨基辛烷客体的分子识别特性(第 2 部分)。十甲基柱[5]芳烃的酸催化合成以良好的收率提供了足够纯的产物,可直接用于后续的 NMR 滴定实验。两节实验针对的是高级化学学生,而 4 小时的大环合成(第 1 节)可能包含在介绍性有机化学实验室课程中。该实验将经典 S E Ar 反应、大环合成、主客体化学、核磁共振波谱和数据处理等概念联系起来,在有机化学合成和超分子化学之间提供了有用的联系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号