当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Complexation of Hexavalent Neptunium(VI) with Oxydiacetic Acid and Its Amide Derivatives in Aqueous Solution: Spectrophotometry and DFT Calculations
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.inorgchem.3c04221 Lei Xu 1, 2 , Xiao-Kun Zhao 3 , Hong Cao 1 , Han-Shi Hu 4 , Jun Li 4 , Jing Chen 1 , Chao Xu 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.inorgchem.3c04221 Lei Xu 1, 2 , Xiao-Kun Zhao 3 , Hong Cao 1 , Han-Shi Hu 4 , Jun Li 4 , Jing Chen 1 , Chao Xu 1
Affiliation
|
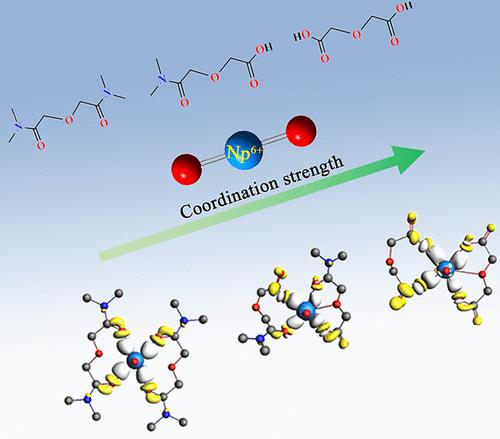
Unfolding the solution coordination chemistry of high-valent transuranium elements with the “CHON”-type ligands is important to understand the fundamental chemistry of actinides and to design more efficient extractants for partitioning of transuranium elements in advanced nuclear fuel cycles. Here, the complexation of a hexavalent neptunyl ion (NpO22+ or Np(VI)) with oxydiacetic acid (ODA) has been systematically investigated in comparison with its amide analogues N,N-dimethyl-3-oxa-glutaramic acid (DMOGA) and N,N,N′,N′-tetramethyl-3-oxa-glutaramide (TMOGA) both experimentally and computationally. The formation of both 1:1 and 1:2 complexes between Np(VI) and the three ligands was identified by spectrophotometry, and their stability constants were obtained and compared with those of hexavalent U(VI) and Pu(VI). The corresponding bonding nature is elucidated by using energy decomposition analysis (EDA), electrostatic potential (ESP), ELF contours, and natural orbitals for chemical valence (NOCV) methods, which shows that the Np–O bonds are essentially ionic in character and the unoccupied 6d orbitals of Np play a key role in enhancing the covalent interactions between Np(VI) and the three ligands.
中文翻译:

六价镎 (VI) 与氧二乙酸及其酰胺衍生物在水溶液中络合:分光光度法和 DFT 计算
揭示高价超铀元素与“CHON”型配体的溶液配位化学对于理解锕系元素的基本化学以及设计更有效的萃取剂用于先进核燃料循环中超铀元素的分配具有重要意义。在此,系统地研究了六价庚烯离子(NpO 2 2+或 Np(VI))与氧二乙酸 (ODA) 的络合,并与其酰胺类似物N , N-二甲基-3-氧杂-戊二酸 (DMOGA ) 进行了比较。) 和N , N , N' , N '-四甲基-3-氧杂-戊二酰胺 (TMOGA) 均通过实验和计算得出。通过分光光度法鉴定了Np(VI)与三种配体之间形成的1:1和1:2配合物,获得了它们的稳定常数并与六价U(VI)和Pu(VI)的稳定常数进行了比较。通过使用能量分解分析(EDA)、静电势(ESP)、ELF 等值线和化学价自然轨道(NOCV)方法阐明了相应的键合性质,这表明 Np-O 键本质上是离子性的,并且Np 的未占据 6d 轨道在增强 Np(VI) 与三个配体之间的共价相互作用方面发挥着关键作用。
更新日期:2024-03-26
中文翻译:

六价镎 (VI) 与氧二乙酸及其酰胺衍生物在水溶液中络合:分光光度法和 DFT 计算
揭示高价超铀元素与“CHON”型配体的溶液配位化学对于理解锕系元素的基本化学以及设计更有效的萃取剂用于先进核燃料循环中超铀元素的分配具有重要意义。在此,系统地研究了六价庚烯离子(NpO 2 2+或 Np(VI))与氧二乙酸 (ODA) 的络合,并与其酰胺类似物N , N-二甲基-3-氧杂-戊二酸 (DMOGA ) 进行了比较。) 和N , N , N' , N '-四甲基-3-氧杂-戊二酰胺 (TMOGA) 均通过实验和计算得出。通过分光光度法鉴定了Np(VI)与三种配体之间形成的1:1和1:2配合物,获得了它们的稳定常数并与六价U(VI)和Pu(VI)的稳定常数进行了比较。通过使用能量分解分析(EDA)、静电势(ESP)、ELF 等值线和化学价自然轨道(NOCV)方法阐明了相应的键合性质,这表明 Np-O 键本质上是离子性的,并且Np 的未占据 6d 轨道在增强 Np(VI) 与三个配体之间的共价相互作用方面发挥着关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号