当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
HOPS-Dependent Endosomal Escape Demands Protein Unfolding
ACS Central Science ( IF 18.2 ) Pub Date : 2024-03-26 , DOI: 10.1021/acscentsci.4c00016 Madeline Zoltek 1 , Angel L. Vázquez Maldonado 2 , Xizi Zhang 2 , Neville Dadina 2 , Lauren Lesiak 2 , Alanna Schepartz 1, 2, 3, 4
ACS Central Science ( IF 18.2 ) Pub Date : 2024-03-26 , DOI: 10.1021/acscentsci.4c00016 Madeline Zoltek 1 , Angel L. Vázquez Maldonado 2 , Xizi Zhang 2 , Neville Dadina 2 , Lauren Lesiak 2 , Alanna Schepartz 1, 2, 3, 4
Affiliation
|
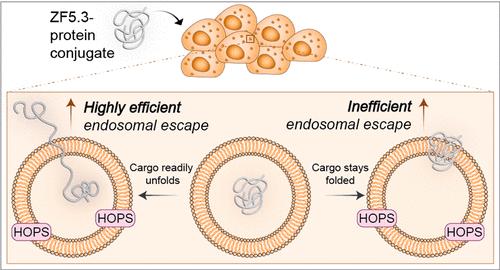
The inefficient translocation of proteins across biological membranes limits their application as potential therapeutics and research tools. In many cases, the translocation of a protein involves two discrete steps: uptake into the endocytic pathway and endosomal escape. Certain charged or amphiphilic molecules can achieve high protein uptake, but few are capable of efficient endosomal escape. One exception to this rule is ZF5.3, a mini-protein that exploits elements of the natural endosomal maturation machinery to translocate across endosomal membranes. Although some ZF5.3–protein conjugates are delivered efficiently to the cytosol or nucleus, overall delivery efficiency varies widely for different cargoes with no obvious design rules. Here we show that delivery efficiency depends on the ability of the cargo to unfold. Using fluorescence correlation spectroscopy, a single-molecule technique that precisely measures intracytosolic protein concentration, we show that regardless of size and pI, low-Tm cargoes of ZF5.3 (including intrinsically disordered domains) bias endosomal escape toward a high-efficiency pathway that requires the homotypic fusion and protein sorting (HOPS) complex. Small protein domains are delivered with moderate efficiency through the same HOPS portal, even if the Tm is high. These findings imply a novel pathway out of endosomes that is exploited by ZF5.3 and provide clear guidance for the selection or design of optimally deliverable therapeutic cargo.
中文翻译:

HOPS 依赖性内体逃逸需要蛋白质展开
蛋白质跨生物膜的低效易位限制了它们作为潜在治疗和研究工具的应用。在许多情况下,蛋白质的易位涉及两个独立的步骤:摄取到内吞途径中和内体逃逸。某些带电或两亲性分子可以实现高蛋白质摄取,但很少有能够有效地逃逸内体。该规则的一个例外是 ZF5.3,它是一种微型蛋白,利用天然内体成熟机制的元件跨内体膜易位。尽管一些 ZF5.3-蛋白缀合物可以有效地递送至细胞质或细胞核,但不同货物的总体递送效率差异很大,没有明显的设计规则。在这里我们表明,交付效率取决于货物展开的能力。使用荧光相关光谱(一种精确测量胞质内蛋白质浓度的单分子技术),我们表明,无论大小和 pI,ZF5.3 的低Tm货物(包括本质上无序的结构域)都会使内体逃逸偏向高效途径这需要同型融合和蛋白质分选 (HOPS) 复合体。即使T m很高,小蛋白质结构域也可以通过相同的 HOPS 门户以中等效率传递。这些发现意味着ZF5.3利用了一条从内体出来的新途径,并为选择或设计最佳可递送治疗货物提供了明确的指导。
更新日期:2024-03-26
中文翻译:

HOPS 依赖性内体逃逸需要蛋白质展开
蛋白质跨生物膜的低效易位限制了它们作为潜在治疗和研究工具的应用。在许多情况下,蛋白质的易位涉及两个独立的步骤:摄取到内吞途径中和内体逃逸。某些带电或两亲性分子可以实现高蛋白质摄取,但很少有能够有效地逃逸内体。该规则的一个例外是 ZF5.3,它是一种微型蛋白,利用天然内体成熟机制的元件跨内体膜易位。尽管一些 ZF5.3-蛋白缀合物可以有效地递送至细胞质或细胞核,但不同货物的总体递送效率差异很大,没有明显的设计规则。在这里我们表明,交付效率取决于货物展开的能力。使用荧光相关光谱(一种精确测量胞质内蛋白质浓度的单分子技术),我们表明,无论大小和 pI,ZF5.3 的低Tm货物(包括本质上无序的结构域)都会使内体逃逸偏向高效途径这需要同型融合和蛋白质分选 (HOPS) 复合体。即使T m很高,小蛋白质结构域也可以通过相同的 HOPS 门户以中等效率传递。这些发现意味着ZF5.3利用了一条从内体出来的新途径,并为选择或设计最佳可递送治疗货物提供了明确的指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号