当前位置:
X-MOL 学术
›
Environ. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Roles of varying carbon chains and functional groups of legacy and emerging per-/polyfluoroalkyl substances in adsorption on metal-organic framework: Insights into mechanism and adsorption prediction
Environmental Research ( IF 8.3 ) Pub Date : 2024-03-20 , DOI: 10.1016/j.envres.2024.118679 Hao Guo , Tongyu Hu , Xiaoman Yang , Zhaoyang Liu , Qianqian Cui , Chenchen Qu , Fayang Guo , Shun Liu , Andrew J. Sweetman , Jingtao Hou , Wenfeng Tan
Environmental Research ( IF 8.3 ) Pub Date : 2024-03-20 , DOI: 10.1016/j.envres.2024.118679 Hao Guo , Tongyu Hu , Xiaoman Yang , Zhaoyang Liu , Qianqian Cui , Chenchen Qu , Fayang Guo , Shun Liu , Andrew J. Sweetman , Jingtao Hou , Wenfeng Tan
|
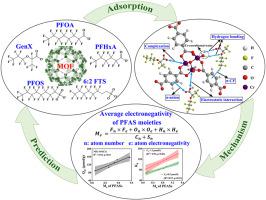
Metal-organic frameworks (MOFs) are promising adsorbents for legacy per-/polyfluoroalkyl substances (PFASs), but they are being replaced by emerging PFASs. The effects of varying carbon chains and functional groups of emerging PFASs on their adsorption behavior on MOFs require attention. This study systematically revealed the structure-adsorption relationships and interaction mechanisms of legacy and emerging PFASs on a typical MOF MIL-101(Cr). It also presented an approach reflecting the average electronegativity of PFAS moieties for adsorption prediction. We demonstrated that short-chain or sulfonate PFASs showed higher adsorption capacities (μmol/g) on MIL-101(Cr) than their long-chain or carboxylate counterparts, respectively. Compared with linear PFASs, their branched isomers were found to exhibit a higher adsorption potential on MIL-101(Cr). In addition, the introduction of ether bond into PFAS molecule (e.g., hexafluoropropylene oxide dimeric acid, GenX) increased the adsorption capacity, while the replacement of CF moieties in PFAS molecule with CH moieties (e.g., 6:2 fluorotelomer sulfonate, 6:2 FTS) caused a decrease in adsorption. Divalent ions (such as Ca and SO) and solution pH have a greater effect on the adsorption of PFASs containing ether bonds or more CF moieties. PFAS adsorption on MIL-101(Cr) was governed by electrostatic interaction, complexation, hydrogen bonding, π-CF interaction, and π-anion interaction as well as steric effects, which were associated with the molecular electronegativity and chain length of each PFAS. The average electronegativity of individual moieties (named ) for each PFAS was estimated and found to show a significantly positive correlation with the corresponding adsorption capacity on MIL-101(Cr). The removal rates of major PFASs in contaminated groundwater by MIL-101(Cr) were also correlated with the corresponding values. These findings will assist with the adsorption prediction for a wide range of PFASs and contribute to tailoring efficient MOF materials.
中文翻译:

传统和新兴全氟烷基/多氟烷基物质的不同碳链和官能团在金属有机框架吸附中的作用:深入了解机制和吸附预测
金属有机框架 (MOF) 是传统全氟/多氟烷基物质 (PFAS) 的有前途的吸附剂,但它们正在被新兴的 PFAS 所取代。新兴 PFAS 的不同碳链和官能团对其在 MOF 上吸附行为的影响需要引起注意。这项研究系统地揭示了传统和新兴 PFAS 在典型 MOF MIL-101(Cr) 上的结构-吸附关系和相互作用机制。它还提出了一种反映 PFAS 部分平均电负性的方法,用于吸附预测。我们证明,短链或磺酸盐 PFAS 在 MIL-101(Cr) 上分别表现出比长链或羧酸盐对应物更高的吸附容量 (μmol/g)。与直链PFAS相比,其支链异构体在MIL-101(Cr)上表现出更高的吸附潜力。此外,在PFAS分子中引入醚键(如六氟环氧丙烷二聚酸,GenX)增加了吸附容量,同时将PFAS分子中的CF部分替换为CH部分(如6:2氟调聚物磺酸盐、6:2 FTS)导致吸附量下降。二价离子(如Ca和SO)和溶液pH值对含有醚键或多CF部分的PFAS的吸附有较大影响。 MIL-101(Cr) 上的 PFAS 吸附受静电相互作用、络合、氢键、π-CF 相互作用、π-阴离子相互作用以及空间效应的控制,这些效应与每种 PFAS 的分子电负性和链长相关。对每个 PFAS 的各个部分(命名为 )的平均电负性进行了估计,发现与 MIL-101(Cr) 上相应的吸附容量呈显着正相关。 MIL-101(Cr)对污染地下水中主要PFAS的去除率也与相应的值相关。这些发现将有助于预测各种 PFAS 的吸附,并有助于定制高效的 MOF 材料。
更新日期:2024-03-20
中文翻译:

传统和新兴全氟烷基/多氟烷基物质的不同碳链和官能团在金属有机框架吸附中的作用:深入了解机制和吸附预测
金属有机框架 (MOF) 是传统全氟/多氟烷基物质 (PFAS) 的有前途的吸附剂,但它们正在被新兴的 PFAS 所取代。新兴 PFAS 的不同碳链和官能团对其在 MOF 上吸附行为的影响需要引起注意。这项研究系统地揭示了传统和新兴 PFAS 在典型 MOF MIL-101(Cr) 上的结构-吸附关系和相互作用机制。它还提出了一种反映 PFAS 部分平均电负性的方法,用于吸附预测。我们证明,短链或磺酸盐 PFAS 在 MIL-101(Cr) 上分别表现出比长链或羧酸盐对应物更高的吸附容量 (μmol/g)。与直链PFAS相比,其支链异构体在MIL-101(Cr)上表现出更高的吸附潜力。此外,在PFAS分子中引入醚键(如六氟环氧丙烷二聚酸,GenX)增加了吸附容量,同时将PFAS分子中的CF部分替换为CH部分(如6:2氟调聚物磺酸盐、6:2 FTS)导致吸附量下降。二价离子(如Ca和SO)和溶液pH值对含有醚键或多CF部分的PFAS的吸附有较大影响。 MIL-101(Cr) 上的 PFAS 吸附受静电相互作用、络合、氢键、π-CF 相互作用、π-阴离子相互作用以及空间效应的控制,这些效应与每种 PFAS 的分子电负性和链长相关。对每个 PFAS 的各个部分(命名为 )的平均电负性进行了估计,发现与 MIL-101(Cr) 上相应的吸附容量呈显着正相关。 MIL-101(Cr)对污染地下水中主要PFAS的去除率也与相应的值相关。这些发现将有助于预测各种 PFAS 的吸附,并有助于定制高效的 MOF 材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号