当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
QbD Approach to Process Characterization and Quantitative Criticality Assessment of Process Parameters†
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2024-03-25 , DOI: 10.1021/acs.oprd.3c00356 Pankaj D. Rege 1 , Andreas Schuster 1 , Jens Lamerz 2 , Christian Moessner 1 , Wolfgang Göhring 1 , Pirmin Hidber 3 , Helmut Stahr 1 , Oana Mihaela Andrei 2 , Janine Burren 2 , Alexandre Moesching 2 , Daniel Coleman 4 , Stefan Hildbrand 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2024-03-25 , DOI: 10.1021/acs.oprd.3c00356 Pankaj D. Rege 1 , Andreas Schuster 1 , Jens Lamerz 2 , Christian Moessner 1 , Wolfgang Göhring 1 , Pirmin Hidber 3 , Helmut Stahr 1 , Oana Mihaela Andrei 2 , Janine Burren 2 , Alexandre Moesching 2 , Daniel Coleman 4 , Stefan Hildbrand 1
Affiliation
|
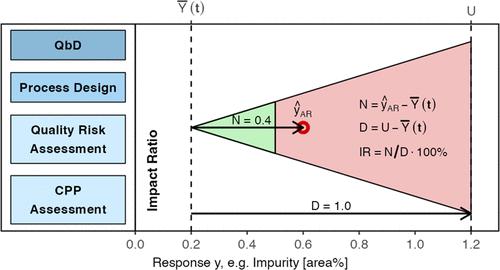
The quality-by-design (QbD) approach is widely utilized for developing and validating manufacturing processes for drug substances as well as drug products. This paper discusses the application of the risk-based QbD approach used at F. Hoffmann-La Roche Ltd. for development, optimization, and characterization of drug substance manufacturing processes for small molecules. It presents the evolution of the QbD concept into statistical thinking and development of a quantitative tool, namely, the impact ratio concept, for its successful implementation. The utilization of this approach is illustrated with a case study from the taselisib drug substance manufacturing process.
中文翻译:

过程表征和过程参数定量关键性评估的 QbD 方法†
质量源于设计 (QbD) 方法广泛用于开发和验证原料药和药品的制造工艺。本文讨论了 F. Hoffmann-La Roche Ltd. 使用的基于风险的 QbD 方法在小分子原料药制造工艺的开发、优化和表征中的应用。它介绍了 QbD 概念向统计思维的演变以及定量工具(即影响比概念)的开发及其成功实施。通过 taselisib 原料药生产过程的案例研究说明了这种方法的利用。
更新日期:2024-03-25
中文翻译:

过程表征和过程参数定量关键性评估的 QbD 方法†
质量源于设计 (QbD) 方法广泛用于开发和验证原料药和药品的制造工艺。本文讨论了 F. Hoffmann-La Roche Ltd. 使用的基于风险的 QbD 方法在小分子原料药制造工艺的开发、优化和表征中的应用。它介绍了 QbD 概念向统计思维的演变以及定量工具(即影响比概念)的开发及其成功实施。通过 taselisib 原料药生产过程的案例研究说明了这种方法的利用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号