当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thiol-Triggered Tandem Dearomative Michael Addition/Intramolecular Henry Reaction of 2-Nitrobenzofurans: Access to Sulfur-Containing Polyheterocyclic Compounds
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-24 , DOI: 10.1021/acs.orglett.4c00646 Jun-Rui Zhuo 1, 2 , Jian-Qiang Zhao 1 , Lei Yang 1 , Yu-Lu Wu 1 , Yan-Ping Zhang 1 , Yong You 1 , Zhen-Hua Wang 1 , Ming-Qiang Zhou 1, 3, 4 , Wei-Cheng Yuan 1, 3, 4
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-24 , DOI: 10.1021/acs.orglett.4c00646 Jun-Rui Zhuo 1, 2 , Jian-Qiang Zhao 1 , Lei Yang 1 , Yu-Lu Wu 1 , Yan-Ping Zhang 1 , Yong You 1 , Zhen-Hua Wang 1 , Ming-Qiang Zhou 1, 3, 4 , Wei-Cheng Yuan 1, 3, 4
Affiliation
|
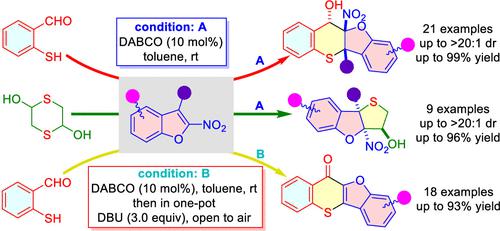
An efficient dearomative cyclization of 2-nitrobenzofurans via a thiol-triggered tandem Michael addition/intramolecular Henry reaction has been developed. A range of thiochromeno[3,2-b]benzofuran-11-ols and tetrahydrothieno[3,2-b]benzofuran-3-ols could be obtained in up to 99% yield and up to >20:1 dr. The valuable thiochromone fused benzofurans could be prepared with the reaction of 2-nitrobenzofurans and 2-mercaptobenzaldehyde via the tandem dearomative Michael addition/intramolecular Henry reaction/rearomatization/oxidative dehydrogenation process in a one-pot two-step operation. A mechanism for the reaction was tentatively proposed.
中文翻译:

硫醇引发的串联脱芳构迈克尔加成/2-硝基苯并呋喃的分子内亨利反应:获得含硫多杂环化合物
通过硫醇触发的串联迈克尔加成/分子内亨利反应,开发了一种有效的 2-硝基苯并呋喃脱芳环化方法。可以获得一系列硫代苯并[3,2- b ]苯并呋喃-11-醇和四氢噻吩并[3,2- b ]苯并呋喃-3-醇,收率高达 99%,dr 高达 >20:1。 2-硝基苯并呋喃与2-巯基苯甲醛通过串联脱芳香迈克尔加成/分子内亨利反应/重芳香化/氧化脱氢一锅两步操作制备有价值的硫色酮稠合苯并呋喃。初步提出了该反应的机理。
更新日期:2024-03-24
中文翻译:

硫醇引发的串联脱芳构迈克尔加成/2-硝基苯并呋喃的分子内亨利反应:获得含硫多杂环化合物
通过硫醇触发的串联迈克尔加成/分子内亨利反应,开发了一种有效的 2-硝基苯并呋喃脱芳环化方法。可以获得一系列硫代苯并[3,2- b ]苯并呋喃-11-醇和四氢噻吩并[3,2- b ]苯并呋喃-3-醇,收率高达 99%,dr 高达 >20:1。 2-硝基苯并呋喃与2-巯基苯甲醛通过串联脱芳香迈克尔加成/分子内亨利反应/重芳香化/氧化脱氢一锅两步操作制备有价值的硫色酮稠合苯并呋喃。初步提出了该反应的机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号