当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical cascade silylation/cyclization of 1,7-dienes to access silyl-substituted benzo[b]azepin-2-ones
Chemical Communications ( IF 4.3 ) Pub Date : 2024-03-25 , DOI: 10.1039/d4cc00499j Zhen Zhang 1 , Lichao Lu 1 , Guiling Li 1 , Xiaoyu Sheng 1 , Yijia Zhang 1 , Lin Yang 1 , Jiaqi Zhao 1 , Lei Xie 2 , Jiazhu Li 1 , Kai Sun 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-03-25 , DOI: 10.1039/d4cc00499j Zhen Zhang 1 , Lichao Lu 1 , Guiling Li 1 , Xiaoyu Sheng 1 , Yijia Zhang 1 , Lin Yang 1 , Jiaqi Zhao 1 , Lei Xie 2 , Jiazhu Li 1 , Kai Sun 1
Affiliation
|
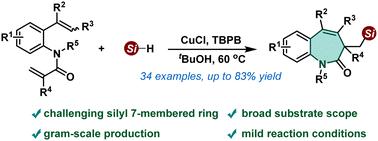
A novel silyl radical-induced cascade silylation/cyclization of 1,7-dienes has been realized employing readily available hydrosilanes as a silicon source and Cu(I) salt as a catalyst. This protocol introduces diverse silicon fragments into a challenging 7-membered ring structure and provides an efficient approach to a wide array of biologically important silyl-substituted benzo[b]azepin-2-ones. Several control experiments suggest that the reaction undergoes a free radical process. The gram-scale synthesis and late-stage transformations further demonstrate the scalability and applicability of the reaction in organic synthesis.
中文翻译:

1,7-二烯的自由基级联甲硅烷基化/环化得到甲硅烷基取代的苯并[b]氮杂-2-酮
采用容易获得的氢硅烷作为硅源和Cu( I )盐作为催化剂,实现了一种新型甲硅烷基诱导的1,7-二烯级联甲硅烷基化/环化。该方案将不同的硅片段引入具有挑战性的七元环结构中,并为广泛的生物学上重要的甲硅烷基取代的苯并[ b ]azepin-2-ones提供了一种有效的方法。一些对照实验表明该反应经历了自由基过程。克级合成和后期转化进一步证明了该反应在有机合成中的可扩展性和适用性。
更新日期:2024-03-25
中文翻译:

1,7-二烯的自由基级联甲硅烷基化/环化得到甲硅烷基取代的苯并[b]氮杂-2-酮
采用容易获得的氢硅烷作为硅源和Cu( I )盐作为催化剂,实现了一种新型甲硅烷基诱导的1,7-二烯级联甲硅烷基化/环化。该方案将不同的硅片段引入具有挑战性的七元环结构中,并为广泛的生物学上重要的甲硅烷基取代的苯并[ b ]azepin-2-ones提供了一种有效的方法。一些对照实验表明该反应经历了自由基过程。克级合成和后期转化进一步证明了该反应在有机合成中的可扩展性和适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号