当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unassisted Photoelectrochemical H2O2 Production with In Situ Glycerol Valorization Using α-Fe2O3
Nano Letters ( IF 10.8 ) Pub Date : 2024-03-25 , DOI: 10.1021/acs.nanolett.3c05136 Sarang Kim 1 , Dongrak Oh 1 , Ji-Wook Jang 1, 2, 3
Nano Letters ( IF 10.8 ) Pub Date : 2024-03-25 , DOI: 10.1021/acs.nanolett.3c05136 Sarang Kim 1 , Dongrak Oh 1 , Ji-Wook Jang 1, 2, 3
Affiliation
|
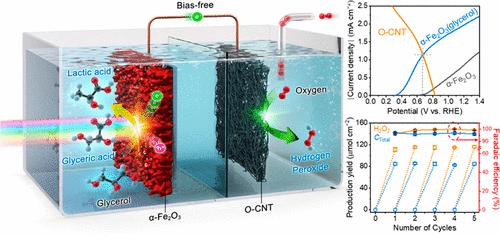
Photoelectrochemical (PEC) H2O2 production via two-electron O2 reduction is promising for H2O2 production without emitting CO2. For PEC H2O2 production, α-Fe2O3 is an ideal semiconductor owing to its earth abundance, superior stability in water, and an appropriate band gap for efficient solar light utilization. Moreover, its conduction band is suitable for O2 reduction to produce H2O2. However, a significant overpotential for water oxidation is required due to the poor surface properties of α-Fe2O3. Thus, unassisted solar H2O2 production is not yet possible. Herein, we demonstrate unassisted PEC H2O2 production using α-Fe2O3 for the first time by applying glycerol oxidation, which requires less bias compared with water oxidation. We obtain maximum Faradaic efficiencies of 96.89 ± 0.6% and 100% for glycerol oxidation and H2O2 production, respectively, with high stability for 25 h. Our results indicate that unassisted and stable PEC H2O2 production is feasible with in situ glycerol valorization using the α-Fe2O3 photoanode.
中文翻译:

使用 α-Fe2O3 原位甘油增值无辅助光电化学生产 H2O2
通过双电子O 2 还原的光电化学(PEC)H 2 O 2 生产有望在不排放CO 2的情况下生产H 2 O 2。对于PEC H 2 O 2生产,α-Fe 2 O 3是一种理想的半导体,因为它在地球上储量丰富,在水中具有优异的稳定性,并且具有适合有效利用太阳光的带隙。而且其导带适合O 2还原生成H 2 O 2。然而,由于α-Fe 2 O 3的表面性质较差,因此需要显着的水氧化过电势。因此,无辅助太阳能生产H 2 O 2尚不可能。在此,我们首次通过应用甘油氧化证明了使用α-Fe 2 O 3进行无辅助PEC H 2 O 2生产,与水氧化相比,这需要更少的偏差。我们获得了甘油氧化和 H 2 O 2生产的最大法拉第效率分别为 96.89 ± 0.6% 和 100% ,并且在 25 小时内具有高稳定性。我们的结果表明,使用 α-Fe 2 O 3光电阳极原位甘油增值,无辅助且稳定的 PEC H 2 O 2生产是可行的。
更新日期:2024-03-25
中文翻译:

使用 α-Fe2O3 原位甘油增值无辅助光电化学生产 H2O2
通过双电子O 2 还原的光电化学(PEC)H 2 O 2 生产有望在不排放CO 2的情况下生产H 2 O 2。对于PEC H 2 O 2生产,α-Fe 2 O 3是一种理想的半导体,因为它在地球上储量丰富,在水中具有优异的稳定性,并且具有适合有效利用太阳光的带隙。而且其导带适合O 2还原生成H 2 O 2。然而,由于α-Fe 2 O 3的表面性质较差,因此需要显着的水氧化过电势。因此,无辅助太阳能生产H 2 O 2尚不可能。在此,我们首次通过应用甘油氧化证明了使用α-Fe 2 O 3进行无辅助PEC H 2 O 2生产,与水氧化相比,这需要更少的偏差。我们获得了甘油氧化和 H 2 O 2生产的最大法拉第效率分别为 96.89 ± 0.6% 和 100% ,并且在 25 小时内具有高稳定性。我们的结果表明,使用 α-Fe 2 O 3光电阳极原位甘油增值,无辅助且稳定的 PEC H 2 O 2生产是可行的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号