当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidative Dehydrogenation of Ethane to Ethylene over Potassium Tungstate Catalysts
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.iecr.3c04549 Duanxing Li 1 , Zenghao Wei 1 , Kazuhiro Takanabe 1, 2
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.iecr.3c04549 Duanxing Li 1 , Zenghao Wei 1 , Kazuhiro Takanabe 1, 2
Affiliation
|
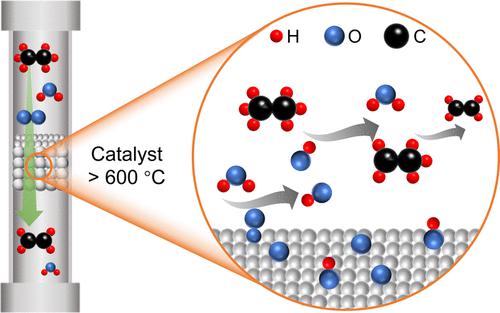
This study elucidated the mechanism of catalytic oxidative dehydrogenation (ODH) of ethane over K2WO4/SiO2 by using microkinetic analysis and simulation with gas-phase chemistry. The K2WO4/SiO2 catalyst prepared with amorphous silica as starting material and cristobalite produced during 900 °C calcination exhibited better performance than catalysts prepared with crystallized silica (cristobalite). The kinetic analysis suggested that both C2H6 and O2 show positive dependences and the reaction proceeds by C–H activation via surface O species (O*, likely peroxide species)-mediated H-abstraction at low conversion. Upon addition of H2O in the reactant stream, the conversion rate drastically improved. Kinetic orders with respective to O2 and H2O pressure are consistent with OH radical formation through the reaction of O* species with H2O, which would then activate the C–H of C2H6 molecules in the gas phase. The experimentally observed C2H6 conversion rate involved contributions from both O*-mediated (dry) and OH radical-mediated (wet) parallel pathways. By assuming that the main kinetic consequence of cofed H2O was generating OH radicals, kinetic simulation illustrated the promotional effect of cofed H2O on C2H6 activation while not affecting the product distribution, which matched well with experimentally observed trends.
中文翻译:

钨酸钾催化剂上乙烷氧化脱氢制乙烯
本研究通过微动力学分析和气相化学模拟,阐明了乙烷在 K 2 WO 4 /SiO 2上催化氧化脱氢(ODH)的机理。以无定形二氧化硅为原料,900 ℃煅烧生成方英石制备的K 2 WO 4 /SiO 2催化剂表现出比结晶二氧化硅(方英石)制备的催化剂更好的性能。动力学分析表明,C 2 H 6和O 2均表现出正依赖性,并且反应通过表面O物种(O*,可能是过氧化物物种)介导的H-抽象在低转化率下通过C-H激活进行。在反应物流中添加H 2 O后,转化率显着提高。相对于O 2和H 2 O压力的动力学顺序与通过O*物质与H 2 O反应形成OH自由基一致,该自由基随后将激活气相中C 2 H 6分子的C-H 。实验观察到的C 2 H 6转化率涉及O*介导(干)和OH自由基介导(湿)平行途径的贡献。通过假设cofed H 2 O的主要动力学后果是产生OH自由基,动力学模拟说明了cofed H 2 O对C 2 H 6活化的促进作用,同时不影响产物分布,这与实验观察到的趋势非常吻合。
更新日期:2024-03-22
中文翻译:

钨酸钾催化剂上乙烷氧化脱氢制乙烯
本研究通过微动力学分析和气相化学模拟,阐明了乙烷在 K 2 WO 4 /SiO 2上催化氧化脱氢(ODH)的机理。以无定形二氧化硅为原料,900 ℃煅烧生成方英石制备的K 2 WO 4 /SiO 2催化剂表现出比结晶二氧化硅(方英石)制备的催化剂更好的性能。动力学分析表明,C 2 H 6和O 2均表现出正依赖性,并且反应通过表面O物种(O*,可能是过氧化物物种)介导的H-抽象在低转化率下通过C-H激活进行。在反应物流中添加H 2 O后,转化率显着提高。相对于O 2和H 2 O压力的动力学顺序与通过O*物质与H 2 O反应形成OH自由基一致,该自由基随后将激活气相中C 2 H 6分子的C-H 。实验观察到的C 2 H 6转化率涉及O*介导(干)和OH自由基介导(湿)平行途径的贡献。通过假设cofed H 2 O的主要动力学后果是产生OH自由基,动力学模拟说明了cofed H 2 O对C 2 H 6活化的促进作用,同时不影响产物分布,这与实验观察到的趋势非常吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号