当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Peptide and Protein Desulfurization with Diboron Reagents
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.orglett.4c00609 Ruiheng Jing 1 , Maciej A. Walczak 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.orglett.4c00609 Ruiheng Jing 1 , Maciej A. Walczak 1
Affiliation
|
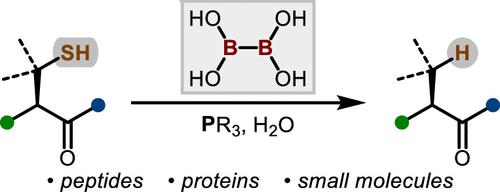
In this Letter, we report a direct and robust desulfurization method employing water-soluble phosphine, specifically tris(2-carboxyethyl)phosphine hydrochloride (TCEP), and tetrahydroxydiboron (B2(OH)4), which serves as a radical initiator. This innovative reaction exhibits compatibility with a diverse array of substrates, including cysteine residues in chemically synthesized oligopeptides and cyclic peptides, alkyl thiols in bioactive molecules, disulfides in commercial proteins, and selenocysteine. We optimized the reaction conditions to minimize the formation of undesired oxidized and borylated byproducts. Furthermore, the refined desulfurization process is executed after native chemical ligation (NCL) in a single pot, streamlining the existing synthetic approaches. This demonstrates its potential applications in the synthesis of complex peptides and proteins, showcasing a significant advancement in the field.
中文翻译:

使用二硼试剂进行肽和蛋白质脱硫
在这封信中,我们报告了一种直接而稳健的脱硫方法,使用水溶性膦,特别是三(2-羧乙基)膦盐酸盐(TCEP)和四羟基二硼(B 2 (OH) 4)作为自由基引发剂。这种创新反应表现出与多种底物的兼容性,包括化学合成寡肽和环肽中的半胱氨酸残基、生物活性分子中的烷基硫醇、商业蛋白质中的二硫化物和硒代半胱氨酸。我们优化了反应条件,以最大限度地减少不需要的氧化和硼化副产物的形成。此外,精炼脱硫过程是在单锅中的天然化学连接(NCL)后进行的,简化了现有的合成方法。这证明了其在复杂肽和蛋白质合成中的潜在应用,展示了该领域的重大进步。
更新日期:2024-03-22
中文翻译:

使用二硼试剂进行肽和蛋白质脱硫
在这封信中,我们报告了一种直接而稳健的脱硫方法,使用水溶性膦,特别是三(2-羧乙基)膦盐酸盐(TCEP)和四羟基二硼(B 2 (OH) 4)作为自由基引发剂。这种创新反应表现出与多种底物的兼容性,包括化学合成寡肽和环肽中的半胱氨酸残基、生物活性分子中的烷基硫醇、商业蛋白质中的二硫化物和硒代半胱氨酸。我们优化了反应条件,以最大限度地减少不需要的氧化和硼化副产物的形成。此外,精炼脱硫过程是在单锅中的天然化学连接(NCL)后进行的,简化了现有的合成方法。这证明了其在复杂肽和蛋白质合成中的潜在应用,展示了该领域的重大进步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号