当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic insight into the degradation of sulfadiazine by electro-Fenton system: Role of different reactive species
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.jhazmat.2024.134063 Yanshi Zheng , Jinyan Yang , Mei Li , Yingshi Zhu , Jiayu Liang , Dehai Yu , Ziyao Wang , Jianchuan Pei
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.jhazmat.2024.134063 Yanshi Zheng , Jinyan Yang , Mei Li , Yingshi Zhu , Jiayu Liang , Dehai Yu , Ziyao Wang , Jianchuan Pei
|
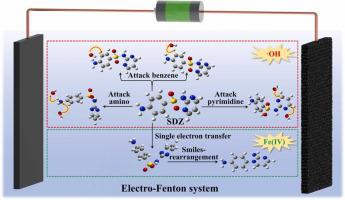
Sulfadiazine (SDZ), a widely used effective antibiotic, is resistant to conventional biological treatment, which is concerning since untreated SDZ discharge can pose a significant environmental risk. Electro-Fenton (EF) technology is a promising advanced oxidation technology for efficiently removing SDZ. However, due to the limitations of traditional experimental methods, there is a lack of in-depth study on the mechanism of ·OH-dominated SDZ degradation in EF process. In this study, an EF system was established for SDZ degradation and the transformation products (TPs) were detected by mass spectrometry. Dynamic thermodynamic, kinetic and wave function analysis of reactants, transition states and intermediates were proposed by density functional theory calculations, which was applied to elucidate the underlying mechanism of SDZ degradation. Experimental results showed that amino, benzene, and pyrimidine sites in SDZ were oxidized by ·OH, producing TPs through hydrogen abstraction and addition reactions. ·OH was kinetically more likely to attack SDZ than SDZ. Fe(IV) dominated the single-electron transfer oxidation reaction of SDZ, and the formed organic radicals can spontaneously generate the de-SO product via Smiles rearrangement. Toxicity experiments showed the toxicity of SDZ and TPs can be greatly reduced. The results of this study promote the understanding of SDZ degradation mechanism in-depth. Sulfadiazine (SDZ) is one of the antibiotics widely used around the world. However, it has posed a significant environmental risk due to its overuse and cannot be efficiently removed by traditional treatment methods. The lack of in-depth study on SDZ degradation mechanism under reactive species limits the improvement of SDZ degradation efficiency. Therefore, this work focused on SDZ degradation mechanism in-depth under electro-Fenton system through reactive species investigation, mass spectrometry analysis, and theoretical calculation. The results in this study can provide a theoretical basis for improving the SDZ degradation efficiency which will contribute to solving SDZ pollution problems.
中文翻译:

电芬顿系统降解磺胺嘧啶的机理研究:不同活性物质的作用
磺胺嘧啶 (SDZ) 是一种广泛使用的有效抗生素,对传统的生物处理具有抵抗力,这一点令人担忧,因为未经处理的 SDZ 排放可能会造成重大的环境风险。电芬顿(EF)技术是一种很有前景的高效去除 SDZ 的高级氧化技术。然而,由于传统实验方法的限制,目前缺乏对EF过程中·OH主导的SDZ降解机理的深入研究。本研究建立了 SDZ 降解的 EF 系统,并通过质谱法检测转化产物(TP)。通过密度泛函理论计算提出了反应物、过渡态和中间体的动态热力学、动力学和波函数分析,用于阐明SDZ降解的潜在机制。实验结果表明,SDZ中的氨基、苯和嘧啶位点被·OH氧化,通过夺氢和加成反应生成TPs。 ·OH 在动力学上比 SDZ 更有可能攻击 SDZ。 Fe(IV)主导SDZ的单电子转移氧化反应,形成的有机自由基可以通过Smiles重排自发生成脱SO产物。毒性实验表明SDZ和TPs的毒性可以大大降低。本研究结果促进了对SDZ降解机制的深入认识。磺胺嘧啶(SDZ)是世界各地广泛使用的抗生素之一。然而,由于其过度使用,它造成了重大的环境风险,并且传统的处理方法无法有效去除。对活性物种下SDZ降解机制缺乏深入研究限制了SDZ降解效率的提高。因此,本工作通过活性组分研究、质谱分析和理论计算,深入研究电Fenton体系下SDZ的降解机理。本研究结果可为提高SDZ降解效率提供理论依据,有助于解决SDZ污染问题。
更新日期:2024-03-19
中文翻译:

电芬顿系统降解磺胺嘧啶的机理研究:不同活性物质的作用
磺胺嘧啶 (SDZ) 是一种广泛使用的有效抗生素,对传统的生物处理具有抵抗力,这一点令人担忧,因为未经处理的 SDZ 排放可能会造成重大的环境风险。电芬顿(EF)技术是一种很有前景的高效去除 SDZ 的高级氧化技术。然而,由于传统实验方法的限制,目前缺乏对EF过程中·OH主导的SDZ降解机理的深入研究。本研究建立了 SDZ 降解的 EF 系统,并通过质谱法检测转化产物(TP)。通过密度泛函理论计算提出了反应物、过渡态和中间体的动态热力学、动力学和波函数分析,用于阐明SDZ降解的潜在机制。实验结果表明,SDZ中的氨基、苯和嘧啶位点被·OH氧化,通过夺氢和加成反应生成TPs。 ·OH 在动力学上比 SDZ 更有可能攻击 SDZ。 Fe(IV)主导SDZ的单电子转移氧化反应,形成的有机自由基可以通过Smiles重排自发生成脱SO产物。毒性实验表明SDZ和TPs的毒性可以大大降低。本研究结果促进了对SDZ降解机制的深入认识。磺胺嘧啶(SDZ)是世界各地广泛使用的抗生素之一。然而,由于其过度使用,它造成了重大的环境风险,并且传统的处理方法无法有效去除。对活性物种下SDZ降解机制缺乏深入研究限制了SDZ降解效率的提高。因此,本工作通过活性组分研究、质谱分析和理论计算,深入研究电Fenton体系下SDZ的降解机理。本研究结果可为提高SDZ降解效率提供理论依据,有助于解决SDZ污染问题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号