当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Continuous Scavenging of Long-Lived Reactive Radicals for Enhancing the Photocatalytic Reduction Efficiency of Conjugated Polymers
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.iecr.3c04141 Qiu Zhuang 1, 2 , Siyao Cheng 2 , Shengke Li 3 , Yaofeng Zhu 4 , Ruru Gao 2 , Lai Jiang 5 , Aming Xie 6 , Wei Dong 2 , Weijin Li 5
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.iecr.3c04141 Qiu Zhuang 1, 2 , Siyao Cheng 2 , Shengke Li 3 , Yaofeng Zhu 4 , Ruru Gao 2 , Lai Jiang 5 , Aming Xie 6 , Wei Dong 2 , Weijin Li 5
Affiliation
|
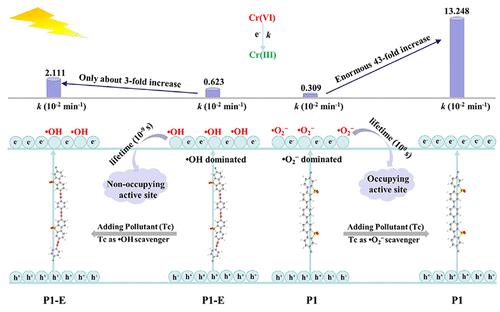
Reactive radicals can significantly affect the timely exposure of the catalyst’s active site and lead to a significant impact on reaction rates, especially for long-lived radicals. Here, two types of metal-free polymers are designed according to the theoretical calculations: one without acetylene, which produces more long-lived superoxide radicals, and another with acetylene, which produces more short-lived hydroxyl radicals. Subsequent experimental characterization revealed that the metal-free polymer containing acetylene (P1-E) outperformed the acetylene-free polymer (P1) in terms of photon absorption, the separation of photogenerated charges, and the photoreduction of heavy metals (Cr(VI)). However, when tetracycline was introduced as a radical scavenger, the continuous consumption of long-lived superoxide radicals resulted in a 43-fold increase in the rate constant (k) of Cr(VI) photoreduction in the P1 system (from 0.00309 to 0.13248 min–1). In contrast, in the P1-E system dominated by short-lived hydroxyl radicals, the rate of Cr(VI) photoreduction increased by a factor of only 3 (from 0.00623 to 0.02111 min–1). This significant transformation can be attributed to the prolonged occupation of active sites on the catalyst surface by long-lived free radicals, thereby inhibiting the efficiency of the reduction reaction. However, for short-lived free radicals, their rapid annihilation exposes more active sites on the catalyst. Therefore, in the coexisting system of tetracycline and Cr(VI), there are distinct differences in the performance of P1 and P1-E catalysts for the photoreduction of Cr(VI). The same phenomenon was also observed with the addition of other contaminants (p-nitrophenol, levofloxacin, and carbamazepine). This study highlights that the removal of long-lived radicals can significantly improve the photocatalytic performance and offers new insights for enhancing the efficiency of photocatalytic reactions.
中文翻译:

连续清除长寿命活性自由基以提高共轭聚合物的光催化还原效率
反应性自由基会显着影响催化剂活性位点的及时暴露,并对反应速率产生显着影响,尤其是长寿命自由基。这里,根据理论计算设计了两种类型的无金属聚合物:一种不含乙炔,会产生更多长寿命的超氧自由基,另一种含有乙炔,会产生更多短寿命的羟基自由基。随后的实验表征表明,含乙炔的无金属聚合物(P1-E)在光子吸收、光生电荷分离和重金属(Cr(VI))的光还原方面优于不含乙炔的聚合物(P1)。 。然而,当引入四环素作为自由基清除剂时,长寿命超氧自由基的持续消耗导致P1系统中Cr(VI)光还原的速率常数( k )增加了43倍(从0.00309分钟增加到0.13248分钟) –1)。相比之下,在以短命羟基自由基为主的 P1-E 体系中,Cr(VI) 光还原速率仅增加了 3 倍(从 0.00623 增加到 0.02111 min –1)。这种显着的转变可归因于长寿命自由基长时间占据催化剂表面的活性位点,从而抑制了还原反应的效率。然而,对于短命自由基,它们的快速消灭会暴露催化剂上更多的活性位点。因此,在四环素和Cr(VI)共存体系中,P1和P1-E催化剂光还原Cr(VI)的性能存在明显差异。添加其他污染物(对硝基苯酚、左氧氟沙星和卡马西平)也观察到相同的现象。这项研究强调,去除长寿命自由基可以显着提高光催化性能,并为提高光催化反应效率提供新的见解。
更新日期:2024-03-22
中文翻译:

连续清除长寿命活性自由基以提高共轭聚合物的光催化还原效率
反应性自由基会显着影响催化剂活性位点的及时暴露,并对反应速率产生显着影响,尤其是长寿命自由基。这里,根据理论计算设计了两种类型的无金属聚合物:一种不含乙炔,会产生更多长寿命的超氧自由基,另一种含有乙炔,会产生更多短寿命的羟基自由基。随后的实验表征表明,含乙炔的无金属聚合物(P1-E)在光子吸收、光生电荷分离和重金属(Cr(VI))的光还原方面优于不含乙炔的聚合物(P1)。 。然而,当引入四环素作为自由基清除剂时,长寿命超氧自由基的持续消耗导致P1系统中Cr(VI)光还原的速率常数( k )增加了43倍(从0.00309分钟增加到0.13248分钟) –1)。相比之下,在以短命羟基自由基为主的 P1-E 体系中,Cr(VI) 光还原速率仅增加了 3 倍(从 0.00623 增加到 0.02111 min –1)。这种显着的转变可归因于长寿命自由基长时间占据催化剂表面的活性位点,从而抑制了还原反应的效率。然而,对于短命自由基,它们的快速消灭会暴露催化剂上更多的活性位点。因此,在四环素和Cr(VI)共存体系中,P1和P1-E催化剂光还原Cr(VI)的性能存在明显差异。添加其他污染物(对硝基苯酚、左氧氟沙星和卡马西平)也观察到相同的现象。这项研究强调,去除长寿命自由基可以显着提高光催化性能,并为提高光催化反应效率提供新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号