当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Adsorption and Desorption of Uranium(VI) Using a Polymeric Adsorbent: A Combined Theoretical and Experimental Approach with Real-Life Alkaline Leach Liquor
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.iecr.3c04314 Abhijit Das 1 , Animesh Jana 2 , Diptendu Das 3 , Sujoy Biswas 4 , Hariharan Seshadri 5 , M.S. Rao 6 , Sirshendu De 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.iecr.3c04314 Abhijit Das 1 , Animesh Jana 2 , Diptendu Das 3 , Sujoy Biswas 4 , Hariharan Seshadri 5 , M.S. Rao 6 , Sirshendu De 1
Affiliation
|
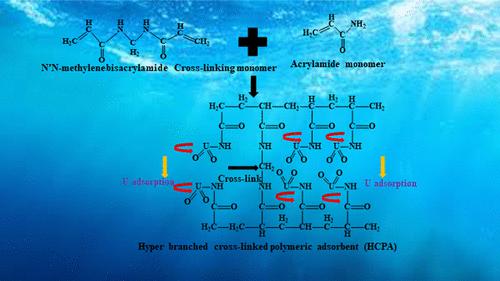
Alkaline leaching is used for the extraction of uranium from rarely available carbonate ore. A hyperbranched cross-linked polymeric adsorbent (HCPA) was developed for selective recovery of uranium(VI) (U) from real-life alkaline leach liquor. HCPA was synthesized using the free radical copolymerization of acrylamide and N, N-methylene bis(acrylamide). In addition, potassium persulfate (as the initiator) and dodecanethiol (as the brancher) were used to transfer the radicals for faster chain formation and smooth branching of the long-chain polymer. Several methods were used extensively to characterize the adsorbent. The Langmuir maximum U adsorption capacity of HCPA was 1012 mg/g at 303 K, and 98% of U was removed from alkaline leach liquor. U was adsorbed at pH 8.5 in the presence of various interfering co-ions and desorbed at pH 11.5 in the form of sodium diuranate (Na2U2O7) precipitate. The adsorption was monolayer, exothermic, and spontaneous in nature. The C–N, CO–NH2, and C–OH groups of HCPA interacted with uranyl ions initiating the coordinative and electrostatic interactions leading to U adsorption. Continuous fixed-bed column runs were performed using the actual leach liquor, and a fundamental kinetic model was used to quantify the performance of columns. The transport parameters were estimated from the model, and scaling-up calculations were performed using these parameters. Five adsorption–desorption cycles were conducted to determine the reusability and structural stability of the synthesized polymeric adsorbent.
中文翻译:

使用聚合物吸附剂有效吸附和解吸铀 (VI):结合实际碱性浸出液的理论和实验方法
碱浸用于从稀有的碳酸盐矿石中提取铀。开发了一种超支化交联聚合物吸附剂 (HCPA),用于从现实生活中的碱性浸出液中选择性回收铀 (VI) (U)。采用丙烯酰胺和N, N-亚甲基双(丙烯酰胺)自由基共聚合成HCPA 。此外,过硫酸钾(作为引发剂)和十二烷硫醇(作为支化剂)用于转移自由基,以加快长链聚合物的链形成和平滑支化。多种方法被广泛用于表征吸附剂。 HCPA在303 K时的Langmuir最大U吸附容量为1012 mg/g,并且从碱性浸出液中去除了98%的U。 U 在各种干扰共离子存在下在 pH 8.5 下被吸附,并在 pH 11.5 下以重铀酸钠 (Na 2 U 2 O 7 ) 沉淀的形式解吸。吸附本质上是单层、放热和自发的。 HCPA 的C-N、CO-NH 2和 C-OH 基团与铀酰离子相互作用,引发配位和静电相互作用,导致 U 吸附。使用实际浸出液进行连续固定床柱运行,并使用基本动力学模型来量化柱的性能。根据模型估计传输参数,并使用这些参数进行放大计算。进行五个吸附-解吸循环以确定合成的聚合物吸附剂的可重复使用性和结构稳定性。
更新日期:2024-03-22
中文翻译:

使用聚合物吸附剂有效吸附和解吸铀 (VI):结合实际碱性浸出液的理论和实验方法
碱浸用于从稀有的碳酸盐矿石中提取铀。开发了一种超支化交联聚合物吸附剂 (HCPA),用于从现实生活中的碱性浸出液中选择性回收铀 (VI) (U)。采用丙烯酰胺和N, N-亚甲基双(丙烯酰胺)自由基共聚合成HCPA 。此外,过硫酸钾(作为引发剂)和十二烷硫醇(作为支化剂)用于转移自由基,以加快长链聚合物的链形成和平滑支化。多种方法被广泛用于表征吸附剂。 HCPA在303 K时的Langmuir最大U吸附容量为1012 mg/g,并且从碱性浸出液中去除了98%的U。 U 在各种干扰共离子存在下在 pH 8.5 下被吸附,并在 pH 11.5 下以重铀酸钠 (Na 2 U 2 O 7 ) 沉淀的形式解吸。吸附本质上是单层、放热和自发的。 HCPA 的C-N、CO-NH 2和 C-OH 基团与铀酰离子相互作用,引发配位和静电相互作用,导致 U 吸附。使用实际浸出液进行连续固定床柱运行,并使用基本动力学模型来量化柱的性能。根据模型估计传输参数,并使用这些参数进行放大计算。进行五个吸附-解吸循环以确定合成的聚合物吸附剂的可重复使用性和结构稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号