当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective sulfonylation to construct 3-sulfonylated oxindoles
Chemical Communications ( IF 4.3 ) Pub Date : 2024-03-22 , DOI: 10.1039/d4cc00802b Hongye Li 1 , Yuqiao Zhou 1 , Zheng Tan 1 , Xiangyu Wang 1 , Yuxin Zhang 2 , Fei Wang 2 , Xiaoming Feng 1 , Xiaohua Liu 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-03-22 , DOI: 10.1039/d4cc00802b Hongye Li 1 , Yuqiao Zhou 1 , Zheng Tan 1 , Xiangyu Wang 1 , Yuxin Zhang 2 , Fei Wang 2 , Xiaoming Feng 1 , Xiaohua Liu 1
Affiliation
|
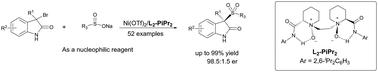
Asymmetric synthesis of 3-sulfonylated 3-substituted oxindoles through the addition of sodium sulfinate salts to 3-bromo-3-substituted oxindoles has been achieved using chiral nickel complexes of N,N′-dioxides. This method facilitates the creation of diverse chiral sulfonyl oxindoles, several of which display promising anticancer properties. Notably, the catalyst demonstrates remarkable tolerance to water, crucial for maintaining enantioselectivity. Furthermore, the utilization of topographic steric maps of the catalysts offers valuable insights into the mechanism underlying enantioselection reversal.
中文翻译:

对映选择性磺酰化构建 3-磺酰化羟吲哚
使用N , N'-二氧化物的手性镍配合物,通过亚磺酸钠盐加成 3-溴-3-取代羟吲哚,实现了 3-磺酰化 3-取代羟吲哚的不对称合成。该方法有助于创建多种手性磺酰基羟吲哚,其中几种具有良好的抗癌特性。值得注意的是,该催化剂表现出显着的耐水性,这对于保持对映选择性至关重要。此外,催化剂的拓扑空间图的利用为对映体选择逆转的机制提供了有价值的见解。
更新日期:2024-03-22
中文翻译:

对映选择性磺酰化构建 3-磺酰化羟吲哚
使用N , N'-二氧化物的手性镍配合物,通过亚磺酸钠盐加成 3-溴-3-取代羟吲哚,实现了 3-磺酰化 3-取代羟吲哚的不对称合成。该方法有助于创建多种手性磺酰基羟吲哚,其中几种具有良好的抗癌特性。值得注意的是,该催化剂表现出显着的耐水性,这对于保持对映选择性至关重要。此外,催化剂的拓扑空间图的利用为对映体选择逆转的机制提供了有价值的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号