当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Atroposelective Construction of Pentatomic Heterobiaryl Diamines through Arylation of 5-Aminoisoxazoles with Azonaphthalenes
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-21 , DOI: 10.1021/acs.orglett.4c00440 Weiwei Luo 1 , Huanhuan Guo 1 , Xueying Qiu 1 , Meijun Ming 2 , Lin Zhang 2 , Hao Zhu 1 , Jun Zhou 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-21 , DOI: 10.1021/acs.orglett.4c00440 Weiwei Luo 1 , Huanhuan Guo 1 , Xueying Qiu 1 , Meijun Ming 2 , Lin Zhang 2 , Hao Zhu 1 , Jun Zhou 1
Affiliation
|
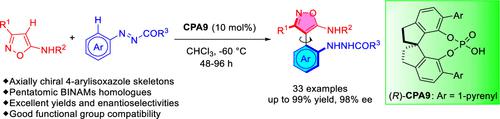
An efficient catalytic asymmetric Michael-type reaction of azonaphthalenes with 5-aminoisoxazoles has been developed. The reaction was based on the utilization of a chiral phosphoric acid as the catalyst, delivering a large panel of axially chiral heterobiaryl diamines in generally good yields with excellent enantioselectivities. The gram-scale reaction and postmodification of the chiral product demonstrated their potentials in the synthesis of chiral catalysts and ligands. This approach not only provides a useful method for the construction of pentatomic heterobiaryl scaffolds but also offers new members to the axially chiral diamine family with promising applications in synthetic and medicinal chemistry.
中文翻译:

通过 5-氨基异恶唑与偶氮萘的芳基化有机催化原子选择性构建五原子杂联二胺
偶氮萘与 5-氨基异恶唑的有效催化不对称迈克尔型反应已被开发出来。该反应基于使用手性磷酸作为催化剂,以良好的产率和优异的对映选择性产生大量轴向手性杂联芳基二胺。手性产物的克级反应和后修饰证明了其在手性催化剂和配体合成中的潜力。这种方法不仅为构建五原子杂联芳基支架提供了一种有用的方法,而且还为轴向手性二胺家族提供了新成员,在合成和药物化学方面具有广阔的应用前景。
更新日期:2024-03-21
中文翻译:

通过 5-氨基异恶唑与偶氮萘的芳基化有机催化原子选择性构建五原子杂联二胺
偶氮萘与 5-氨基异恶唑的有效催化不对称迈克尔型反应已被开发出来。该反应基于使用手性磷酸作为催化剂,以良好的产率和优异的对映选择性产生大量轴向手性杂联芳基二胺。手性产物的克级反应和后修饰证明了其在手性催化剂和配体合成中的潜力。这种方法不仅为构建五原子杂联芳基支架提供了一种有用的方法,而且还为轴向手性二胺家族提供了新成员,在合成和药物化学方面具有广阔的应用前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号