当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Expanding Complex Morpholines Using Systematic Chemical Diversity
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.orglett.4c00528 Sunny Ann Tang 1 , Afton Fults 2 , Shelton R. Boyd 1 , Nikhil Gattu 2 , Kevin A. Tran 3 , Jiayi Fan 1 , Kevin R. MacKenzie 1, 3 , Timothy Palzkill 1 , Damian W. Young 1, 3 , Srinivas Chamakuri 3
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.orglett.4c00528 Sunny Ann Tang 1 , Afton Fults 2 , Shelton R. Boyd 1 , Nikhil Gattu 2 , Kevin A. Tran 3 , Jiayi Fan 1 , Kevin R. MacKenzie 1, 3 , Timothy Palzkill 1 , Damian W. Young 1, 3 , Srinivas Chamakuri 3
Affiliation
|
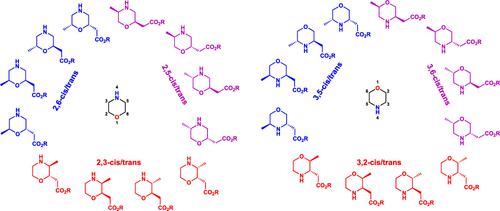
The morpholine heterocycle is a structural unit found in many bioactive compounds and FDA-approved drugs, but the generation of more complex C-functionalized morpholine derivatives remains considerably underexplored. Using systematic chemical diversity (SCD), a concept that guides the expansion of saturated drug-like scaffolds through regiochemical and stereochemical variation, we describe the synthesis of a collection of methyl-substituted morpholine acetic acid esters starting from enantiomerically pure amino acids and amino alcohols. In total, 24 diverse substituted morpholines were produced that vary systematically in regiochemistry and stereochemistry (relative and absolute). These diverse C-substituted morpholines can be directly applied in fragment screening or incorporated as building blocks in medicinal chemistry and library synthesis.
中文翻译:

利用系统化学多样性扩展复杂吗啉
吗啉杂环是许多生物活性化合物和 FDA 批准的药物中发现的结构单元,但更复杂的 C 功能化吗啉衍生物的生成仍远未得到充分探索。利用系统化学多样性(SCD)这一概念,通过区域化学和立体化学变化指导饱和类药物支架的扩展,我们描述了从对映体纯氨基酸和氨基醇开始合成一系列甲基取代吗啉乙酸酯。总共产生了 24 种不同的取代吗啉,它们在区域化学和立体化学(相对和绝对)上系统地变化。这些不同的 C 取代吗啉可直接应用于片段筛选或作为药物化学和文库合成的构建模块。
更新日期:2024-03-22
中文翻译:

利用系统化学多样性扩展复杂吗啉
吗啉杂环是许多生物活性化合物和 FDA 批准的药物中发现的结构单元,但更复杂的 C 功能化吗啉衍生物的生成仍远未得到充分探索。利用系统化学多样性(SCD)这一概念,通过区域化学和立体化学变化指导饱和类药物支架的扩展,我们描述了从对映体纯氨基酸和氨基醇开始合成一系列甲基取代吗啉乙酸酯。总共产生了 24 种不同的取代吗啉,它们在区域化学和立体化学(相对和绝对)上系统地变化。这些不同的 C 取代吗啉可直接应用于片段筛选或作为药物化学和文库合成的构建模块。



























 京公网安备 11010802027423号
京公网安备 11010802027423号