当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Native Amino Group Directed Meta-Selective C–H Arylation of Primary Amines Via Pd/Norbornene Catalysis
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.orglett.4c00721 Shasha Zhang 1 , Gong Zhang 1 , Jie Wang 1 , Yueyao Feng 1 , Zemin Zhang 2 , Si Xie 1 , Ziying Lin 1 , Shiling Yang 1 , Jin Lin 2 , Hua Lin 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.orglett.4c00721 Shasha Zhang 1 , Gong Zhang 1 , Jie Wang 1 , Yueyao Feng 1 , Zemin Zhang 2 , Si Xie 1 , Ziying Lin 1 , Shiling Yang 1 , Jin Lin 2 , Hua Lin 1
Affiliation
|
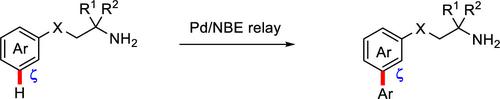
The selective functionalization of remote C–H bonds in free primary amines holds significant promise for the late-stage diversification of pharmaceuticals. However, to date, the direct functionalization of the meta position of amine substrates lacking additional directing groups remains underexplored. In this Letter, we present a successful meta-C–H arylation of free primary amine derivatives using aryl iodides, resulting in synthetically valuable yields. This meta-selective C–H functionalization is achieved through a sequence involving native amino-directed Pd-catalyzed seven-membered cyclometalation, followed by the utilization of a norbornene-type transient mediator.
中文翻译:

通过 Pd/降冰片烯催化,天然氨基定向伯胺间位选择性 C–H 芳基化
游离伯胺中远程 C-H 键的选择性官能化为药物的后期多样化带来了巨大的希望。然而,迄今为止,缺乏额外导向基团的胺底物间位的直接官能化仍未得到充分探索。在这封信中,我们展示了使用芳基碘化物对游离伯胺衍生物进行成功的间位-C-H芳基化,从而获得了具有合成价值的产率。这种元选择性 C-H 功能化是通过涉及天然氨基定向 Pd 催化的七元环金属化的序列实现的,然后利用降冰片烯型瞬时介体。
更新日期:2024-03-20
中文翻译:

通过 Pd/降冰片烯催化,天然氨基定向伯胺间位选择性 C–H 芳基化
游离伯胺中远程 C-H 键的选择性官能化为药物的后期多样化带来了巨大的希望。然而,迄今为止,缺乏额外导向基团的胺底物间位的直接官能化仍未得到充分探索。在这封信中,我们展示了使用芳基碘化物对游离伯胺衍生物进行成功的间位-C-H芳基化,从而获得了具有合成价值的产率。这种元选择性 C-H 功能化是通过涉及天然氨基定向 Pd 催化的七元环金属化的序列实现的,然后利用降冰片烯型瞬时介体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号