当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electroreductive Remote Benzylic C(sp3)–H Arylation of Aliphatic Ethers Using Cyanoarenes for the Synthesis of α-(Hetero)aryl Ethers
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.orglett.4c00615 Liang Zeng 1, 2, 3 , Hua-Zhan Ren 2 , Gui-Fen Lv 1 , Xuan-Hui Ouyang 1 , De-Liang He 3 , Jin-Heng Li 1, 2, 3, 4
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.orglett.4c00615 Liang Zeng 1, 2, 3 , Hua-Zhan Ren 2 , Gui-Fen Lv 1 , Xuan-Hui Ouyang 1 , De-Liang He 3 , Jin-Heng Li 1, 2, 3, 4
Affiliation
|
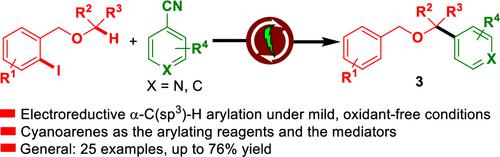
An iodoarene-driven electroreductive remote C(sp3)–H arylation of unsymmetrical 1-(o-iodoaryl)alkyl ethers with cyanoarenes for the site selective synthesis of α-(hetero)aryl ethers is developed. With the introduction of cyanoarenes as both aryl sources and electron transfer mediators, this method includes an iodoarene-driven strategy to enable the regiocontrollable formation of two new bonds, one C(sp2)–H bond, and one C(sp2)–C(sp3) bond, in a single reaction step through the sequence of halogen atom transfer (XAT), hydrogen atom transfer (HAT), radical–radical coupling, and decyanation.
中文翻译:

使用氰基芳烃对脂肪族醚进行电还原远程苯甲基 C(sp3)–H 芳基化以合成 α-(杂)芳基醚
开发了一种碘芳烃驱动的不对称1-(邻碘芳基)烷基醚与氰基芳烃的远程电还原C(sp 3 )–H芳基化反应,用于α-(杂)芳基醚的位点选择性合成。通过引入氰基芳烃作为芳基源和电子转移介体,该方法包括一种碘芳烃驱动策略,能够区域可控地形成两个新键,一个 C(sp 2 )–H 键和一个 C(sp 2 )– C(sp 3 ) 键,在一个反应步骤中通过卤素原子转移 (XAT)、氢原子转移 (HAT)、自由基-自由基偶联和脱氰化的顺序。
更新日期:2024-03-19
中文翻译:

使用氰基芳烃对脂肪族醚进行电还原远程苯甲基 C(sp3)–H 芳基化以合成 α-(杂)芳基醚
开发了一种碘芳烃驱动的不对称1-(邻碘芳基)烷基醚与氰基芳烃的远程电还原C(sp 3 )–H芳基化反应,用于α-(杂)芳基醚的位点选择性合成。通过引入氰基芳烃作为芳基源和电子转移介体,该方法包括一种碘芳烃驱动策略,能够区域可控地形成两个新键,一个 C(sp 2 )–H 键和一个 C(sp 2 )– C(sp 3 ) 键,在一个反应步骤中通过卤素原子转移 (XAT)、氢原子转移 (HAT)、自由基-自由基偶联和脱氰化的顺序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号