当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A neoteric antibacterial ceria-silver nanozyme for abiotic surfaces
Biomaterials ( IF 12.8 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.biomaterials.2024.122527 Abinaya Sindu Pugazhendhi 1 , Craig J Neal 2 , Khoa Minh Ta 3 , Marco Molinari 3 , Udit Kumar 2 , Fei Wei 1 , Elayaraja Kolanthai 2 , Andrew Ady 1 , Christina Drake 4 , Megan Hughes 5 , Shibu Yooseph 6 , Sudipta Seal 7 , Melanie J Coathup 1
Biomaterials ( IF 12.8 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.biomaterials.2024.122527 Abinaya Sindu Pugazhendhi 1 , Craig J Neal 2 , Khoa Minh Ta 3 , Marco Molinari 3 , Udit Kumar 2 , Fei Wei 1 , Elayaraja Kolanthai 2 , Andrew Ady 1 , Christina Drake 4 , Megan Hughes 5 , Shibu Yooseph 6 , Sudipta Seal 7 , Melanie J Coathup 1
Affiliation
|
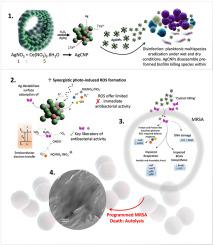
Community-associated and hospital-acquired infections caused by bacteria continue to yield major global challenges to human health. Bacterial contamination on abiotic surfaces is largely spread via high-touch surfaces and contemporary standard disinfection practices show limited efficacy, resulting in unsatisfactory therapeutic outcomes. New strategies that offer non-specific and broad protection are urgently needed. Herein, we report our novel ceria-silver nanozyme engineered at a molar ratio of 5:1 and with a higher trivalent (Ce3+ ) surface fraction. Our results reveal potent levels of surface catalytic activity on both wet and dry surfaces, with rapid, and complete eradication of Pseudomonas aeruginosa , Staphylococcus aureus , and methicillin resistant S. aureus , in both planktonic and biofilm form. Preferential electrostatic adherence of anionic bacteria to the cationic nanozyme surface leads to a catastrophic loss in both aerobic and anaerobic respiration, DNA damage, osmodysregulation, and finally, programmed bacterial lysis. Our data reveal several unique mechanistic avenues of synergistic ceria-Ag efficacy. Ag potentially increases the presence of Ce3+ sites at the ceria-Ag interface, thereby facilitating the formation of harmful H2 O2 , followed by likely permeation across the cell wall. Further, a weakened Ag-induced Ce–O bond may drive electron transfer from the Ec 2 , thereby further facilitating the selective reduction of O2 toward H2 O2 formation. Ag destabilizes the surface adsorption of molecular H2 O2 , potentially leading to higher concentrations of free H2 O2 adjacent to bacteria. To this end, our results show that H2 O2 and/or NO/NO2 − /NO3 − are the key liberators of antibacterial activity, with a limited immediate role being offered by nanozyme-induced ROS including O2 •- and OH• , and likely other light-activated radicals. A mini-pilot proof-of-concept study performed in a pediatric dental clinic setting confirms residual, and continual nanozyme antibacterial efficacy over a 28-day period. These findings open a new approach to alleviate infections caused by bacteria for use on high-touch hard surfaces.
中文翻译:

一种用于非生物表面的新型抗菌二氧化铈银纳米酶
由细菌引起的社区相关感染和医院获得性感染继续给人类健康带来重大的全球挑战。非生物表面上的细菌污染主要通过高接触表面传播,而当代标准消毒实践的效果有限,导致治疗结果不令人满意。迫切需要提供非具体和广泛保护的新战略。在此,我们报告了我们的新型二氧化铈-银纳米酶,其摩尔比为 5:1,具有更高的三价 (Ce3+) 表面分数。我们的结果揭示了在潮湿和干燥表面上的有效表面催化活性水平,能够快速、彻底地根除浮游和生物膜形式的铜绿假单胞菌、金黄色葡萄球菌和耐甲氧西林金黄色葡萄球菌。阴离子细菌优先静电粘附到阳离子纳米酶表面,导致有氧和无氧呼吸的灾难性损失、DNA损伤、渗透调节失调,以及最终的程序性细菌裂解。我们的数据揭示了二氧化铈-银协同功效的几种独特的机制途径。 Ag 可能增加二氧化铈-Ag 界面处 Ce3+ 位点的存在,从而促进有害 H2O2 的形成,随后可能渗透穿过细胞壁。此外,Ag诱导的Ce-O键减弱可能会驱动电子从Ec带转移到O2,从而进一步促进O2选择性还原成H2O2。 Ag 破坏分子 H2O2 的表面吸附,可能导致细菌附近游离 H2O2 浓度较高。 为此,我们的结果表明,H2O2 和/或 NO/NO2−/NO3− 是抗菌活性的关键释放剂,纳米酶诱导的 ROS(包括 O2•- 和 OH• 以及可能的其他物质)提供的直接作用有限。光激活自由基。在儿科牙科诊所进行的一项小型试点概念验证研究证实了纳米酶在 28 天的时间内具有残留且持续的抗菌功效。这些发现开辟了一种新方法,可以减轻高接触硬表面上细菌引起的感染。
更新日期:2024-03-18
中文翻译:

一种用于非生物表面的新型抗菌二氧化铈银纳米酶
由细菌引起的社区相关感染和医院获得性感染继续给人类健康带来重大的全球挑战。非生物表面上的细菌污染主要通过高接触表面传播,而当代标准消毒实践的效果有限,导致治疗结果不令人满意。迫切需要提供非具体和广泛保护的新战略。在此,我们报告了我们的新型二氧化铈-银纳米酶,其摩尔比为 5:1,具有更高的三价 (Ce3+) 表面分数。我们的结果揭示了在潮湿和干燥表面上的有效表面催化活性水平,能够快速、彻底地根除浮游和生物膜形式的铜绿假单胞菌、金黄色葡萄球菌和耐甲氧西林金黄色葡萄球菌。阴离子细菌优先静电粘附到阳离子纳米酶表面,导致有氧和无氧呼吸的灾难性损失、DNA损伤、渗透调节失调,以及最终的程序性细菌裂解。我们的数据揭示了二氧化铈-银协同功效的几种独特的机制途径。 Ag 可能增加二氧化铈-Ag 界面处 Ce3+ 位点的存在,从而促进有害 H2O2 的形成,随后可能渗透穿过细胞壁。此外,Ag诱导的Ce-O键减弱可能会驱动电子从Ec带转移到O2,从而进一步促进O2选择性还原成H2O2。 Ag 破坏分子 H2O2 的表面吸附,可能导致细菌附近游离 H2O2 浓度较高。 为此,我们的结果表明,H2O2 和/或 NO/NO2−/NO3− 是抗菌活性的关键释放剂,纳米酶诱导的 ROS(包括 O2•- 和 OH• 以及可能的其他物质)提供的直接作用有限。光激活自由基。在儿科牙科诊所进行的一项小型试点概念验证研究证实了纳米酶在 28 天的时间内具有残留且持续的抗菌功效。这些发现开辟了一种新方法,可以减轻高接触硬表面上细菌引起的感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号