当前位置:
X-MOL 学术
›
Clin. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Upadacitinib Reduces Crohn’s Disease Symptoms Within the First Week of Induction Therapy
Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2024-03-15 , DOI: 10.1016/j.cgh.2024.02.027 Jean-Frédéric Colombel , Tadakazu Hisamatsu , Raja Atreya , Francesca Bresso , Lena Thin , Remo Panaccione , Rogério Serafim Parra , Sharanya Ford , Valencia P. Remple , Ana Paula Lacerda , Samuel I. Anyanwu , Madhuja Mallick , Andrew Garrison , Miguel Regueiro
Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2024-03-15 , DOI: 10.1016/j.cgh.2024.02.027 Jean-Frédéric Colombel , Tadakazu Hisamatsu , Raja Atreya , Francesca Bresso , Lena Thin , Remo Panaccione , Rogério Serafim Parra , Sharanya Ford , Valencia P. Remple , Ana Paula Lacerda , Samuel I. Anyanwu , Madhuja Mallick , Andrew Garrison , Miguel Regueiro
|
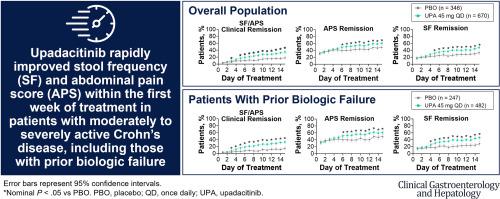
Upadacitinib (UPA), an oral Janus kinase inhibitor, is approved to treat moderately to severely active Crohn’s disease (CD). Because symptomatic response is an important initial treatment goal for patients, we evaluated the rapidity of symptomatic improvement in patients with CD receiving UPA 45 mg once daily (UPA45) induction therapy. This post hoc analysis included pooled data from 2 phase 3, multicenter, double-blind, 12-week induction trials (U-EXCEL and U-EXCEED) and 1 maintenance trial (U-ENDURE). Daily diary data for the first 15 days of UPA45 or placebo (PBO) treatment were used to analyze improvement in very soft/liquid stool frequency (SF) and abdominal pain score (APS). Clinical outcomes were evaluated at every study visit. Overall, 1021 patients (n = 674 UPA45; n = 347 PBO) were analyzed. UPA45 demonstrated greater efficacy vs PBO for SF <3 and APS ≤1, providing rapid relief by day 5 or 6, regardless of prior biologic exposure. Mean changes in SF and APS were greater with UPA45 beginning at week 2 (−2.0 and −0.5, respectively; < .001) and were maintained through week 12 (−3.0 and −1.0, respectively; < .001) vs PBO. The first achievement of daily SF/APS clinical remission occurred earlier with UPA45 (median, 13 d) vs PBO (median, 32 d), and patients treated with UPA45 showed improved rates of SF/APS clinical remission (21.1% UPA45 vs 8.9% PBO) and clinical response (58.8% UPA45 vs 37.9% PBO) starting at week 2 (both ≤ .01). UPA45 provided rapid relief of clinical symptoms within the first week of treatment in patients with CD. numbers: NCT03345849, NCT03345836, and NCT03345823.
中文翻译:

乌帕替尼在诱导治疗的第一周内减轻克罗恩病症状
Upadacitinib (UPA) 是一种口服 Janus 激酶抑制剂,被批准用于治疗中度至重度活动性克罗恩病 (CD)。由于症状缓解是患者重要的初始治疗目标,因此我们评估了接受 UPA 45 mg 每日一次 (UPA45) 诱导治疗的 CD 患者症状改善的速度。这项事后分析包括 2 项 3 期、多中心、双盲、12 周诱导试验(U-EXCEL 和 U-EXCEED)和 1 项维持试验(U-ENDURE)的汇总数据。 UPA45 或安慰剂 (PBO) 治疗前 15 天的每日日记数据用于分析极软/液体粪便频率 (SF) 和腹痛评分 (APS) 的改善。每次研究访问时都会评估临床结果。总体而言,对 1021 名患者(n = 674 UPA45;n = 347 PBO)进行了分析。对于 SF <3 和 APS ≤1,UPA45 表现出比 PBO 更好的功效,无论先前的生物制剂暴露如何,都能在第 5 天或第 6 天快速缓解。与 PBO 相比,从第 2 周开始使用 UPA45 时,SF 和 APS 的平均变化更大(分别为 -2.0 和 -0.5;< .001),并且一直维持到第 12 周(分别为 -3.0 和 -1.0;< .001)。每日 SF/APS 临床缓解的首次实现,UPA45(中位,13 天)比 PBO(中位,32 天)更早,并且接受 UPA45 治疗的患者显示出 SF/APS 临床缓解率的改善(21.1% UPA45 vs 8.9%) PBO)和从第 2 周开始的临床反应(58.8% UPA45 vs 37.9% PBO)(均≤ .01)。 UPA45 在 CD 患者治疗的第一周内迅速缓解了临床症状。编号:NCT03345849、NCT03345836 和 NCT03345823。
更新日期:2024-03-15
中文翻译:

乌帕替尼在诱导治疗的第一周内减轻克罗恩病症状
Upadacitinib (UPA) 是一种口服 Janus 激酶抑制剂,被批准用于治疗中度至重度活动性克罗恩病 (CD)。由于症状缓解是患者重要的初始治疗目标,因此我们评估了接受 UPA 45 mg 每日一次 (UPA45) 诱导治疗的 CD 患者症状改善的速度。这项事后分析包括 2 项 3 期、多中心、双盲、12 周诱导试验(U-EXCEL 和 U-EXCEED)和 1 项维持试验(U-ENDURE)的汇总数据。 UPA45 或安慰剂 (PBO) 治疗前 15 天的每日日记数据用于分析极软/液体粪便频率 (SF) 和腹痛评分 (APS) 的改善。每次研究访问时都会评估临床结果。总体而言,对 1021 名患者(n = 674 UPA45;n = 347 PBO)进行了分析。对于 SF <3 和 APS ≤1,UPA45 表现出比 PBO 更好的功效,无论先前的生物制剂暴露如何,都能在第 5 天或第 6 天快速缓解。与 PBO 相比,从第 2 周开始使用 UPA45 时,SF 和 APS 的平均变化更大(分别为 -2.0 和 -0.5;< .001),并且一直维持到第 12 周(分别为 -3.0 和 -1.0;< .001)。每日 SF/APS 临床缓解的首次实现,UPA45(中位,13 天)比 PBO(中位,32 天)更早,并且接受 UPA45 治疗的患者显示出 SF/APS 临床缓解率的改善(21.1% UPA45 vs 8.9%) PBO)和从第 2 周开始的临床反应(58.8% UPA45 vs 37.9% PBO)(均≤ .01)。 UPA45 在 CD 患者治疗的第一周内迅速缓解了临床症状。编号:NCT03345849、NCT03345836 和 NCT03345823。



























 京公网安备 11010802027423号
京公网安备 11010802027423号