当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extracellular vesicles derived from Lactobacillus johnsonii promote gut barrier homeostasis by enhancing M2 macrophage polarization
Journal of Advanced Research ( IF 10.7 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.jare.2024.03.011 Shiyu Tao , Jinping Fan , Jingjing Li , Zhifeng Wu , Yong Yao , Zhenyu Wang , Yujun Wu , Xiangdong Liu , Yingping Xiao , Hong Wei
Journal of Advanced Research ( IF 10.7 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.jare.2024.03.011 Shiyu Tao , Jinping Fan , Jingjing Li , Zhifeng Wu , Yong Yao , Zhenyu Wang , Yujun Wu , Xiangdong Liu , Yingping Xiao , Hong Wei
|
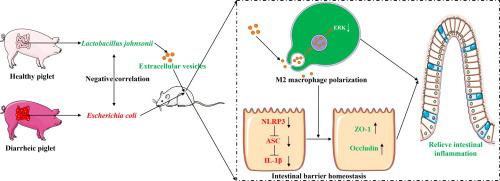
Diarrheic disease is a common intestinal health problem worldwide, causing great suffering to humans and animals. Precise manipulation strategies based on probiotics to combat diarrheic diseases have not been fully developed. The aim of this study was to investigate the molecular mechanisms by which probiotics manipulate macrophage against diarrheic disease. Metagenome reveals gut microbiome profiles of healthy and diarrheic piglets. Fecal microbial transplantation (FMT) was employed to explore the causal relationship between gut microbes and diarrhea. The protective role of probiotics and their derived extracellular vesicles (EVs) was investigated in ETEC K88-infected mice. Macrophage depletion was performed to assess the role of macrophages in EVs against diarrhea. Execution of cell co-culture and transcriptome analyses elucidated the molecular mechanisms by which EVs modulate the macrophage and intestinal epithelial barrier. was enriched in weaned diarrheic piglets, while () showed a negative correlation with . The transmission of diarrheic illness symptoms was achieved by transferring fecal microbiota, but not metabolites, from diarrheic pigs to germ-free (GF) mice. intervention prevented the transmission of disease phenotypes from diarrheic piglets to GF mice. also reduces the gut inflammation induced by ETEC K88. The EVs secreted by demonstrated enhanced efficacy in mitigating the adverse impacts induced by ETEC K88 through the modulation of macrophage phenotype. experiments have revealed that EVs activate M2 macrophages in a manner that shuts down ERK, thereby inhibiting NLRP3 activation in intestinal epithelial cells. Our results reveal that intestinal microbiota drives the onset of diarrheic disease and that probiotic-derived EVs ameliorate diarrheic disease symptoms by modulating macrophage phenotypes. These findings can enhance the advancement of innovative therapeutic approaches for diarrheic conditions based on probiotic-derived EVs.
中文翻译:

约氏乳杆菌衍生的细胞外囊泡通过增强 M2 巨噬细胞极化来促进肠道屏障稳态
腹泻病是世界范围内常见的肠道健康问题,给人类和动物带来巨大痛苦。基于益生菌对抗腹泻病的精确操作策略尚未完全开发。本研究的目的是研究益生菌操纵巨噬细胞对抗腹泻病的分子机制。宏基因组揭示了健康和腹泻仔猪的肠道微生物组特征。采用粪便微生物移植(FMT)来探索肠道微生物与腹泻之间的因果关系。在 ETEC K88 感染的小鼠中研究了益生菌及其衍生的细胞外囊泡 (EV) 的保护作用。进行巨噬细胞耗竭是为了评估巨噬细胞在 EV 中抗腹泻的作用。细胞共培养和转录组分析的执行阐明了 EV 调节巨噬细胞和肠上皮屏障的分子机制。断奶腹泻仔猪中富集,而()与 呈负相关。腹泻疾病症状的传播是通过将腹泻猪的粪便微生物群(而非代谢物)转移到无菌(GF)小鼠身上来实现的。干预措施阻止了疾病表型从腹泻仔猪传播给 GF 小鼠。还可以减少 ETEC K88 引起的肠道炎症。通过调节巨噬细胞表型,分泌的 EV 可以增强减轻 ETEC K88 引起的不利影响的功效。实验表明,EVs 以关闭 ERK 的方式激活 M2 巨噬细胞,从而抑制肠上皮细胞中 NLRP3 的激活。我们的结果表明,肠道微生物群驱动腹泻病的发作,而益生菌衍生的 EV 通过调节巨噬细胞表型来改善腹泻病症状。这些发现可以促进基于益生菌衍生的 EV 的腹泻病创新治疗方法的进步。
更新日期:2024-03-18
中文翻译:

约氏乳杆菌衍生的细胞外囊泡通过增强 M2 巨噬细胞极化来促进肠道屏障稳态
腹泻病是世界范围内常见的肠道健康问题,给人类和动物带来巨大痛苦。基于益生菌对抗腹泻病的精确操作策略尚未完全开发。本研究的目的是研究益生菌操纵巨噬细胞对抗腹泻病的分子机制。宏基因组揭示了健康和腹泻仔猪的肠道微生物组特征。采用粪便微生物移植(FMT)来探索肠道微生物与腹泻之间的因果关系。在 ETEC K88 感染的小鼠中研究了益生菌及其衍生的细胞外囊泡 (EV) 的保护作用。进行巨噬细胞耗竭是为了评估巨噬细胞在 EV 中抗腹泻的作用。细胞共培养和转录组分析的执行阐明了 EV 调节巨噬细胞和肠上皮屏障的分子机制。断奶腹泻仔猪中富集,而()与 呈负相关。腹泻疾病症状的传播是通过将腹泻猪的粪便微生物群(而非代谢物)转移到无菌(GF)小鼠身上来实现的。干预措施阻止了疾病表型从腹泻仔猪传播给 GF 小鼠。还可以减少 ETEC K88 引起的肠道炎症。通过调节巨噬细胞表型,分泌的 EV 可以增强减轻 ETEC K88 引起的不利影响的功效。实验表明,EVs 以关闭 ERK 的方式激活 M2 巨噬细胞,从而抑制肠上皮细胞中 NLRP3 的激活。我们的结果表明,肠道微生物群驱动腹泻病的发作,而益生菌衍生的 EV 通过调节巨噬细胞表型来改善腹泻病症状。这些发现可以促进基于益生菌衍生的 EV 的腹泻病创新治疗方法的进步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号