当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective Oxypalladation of (o-Alkynylaryl)amide-Triggered Site-Selective C−H Annulation for Stereoselective Synthesis of Succinimide-Fused Polycycles
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-19 , DOI: 10.1002/adsc.202301367 Dattatri V 1 , Jagadeesh Babu Nanubolu 1 , Maddi Sridhar Reddy 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-19 , DOI: 10.1002/adsc.202301367 Dattatri V 1 , Jagadeesh Babu Nanubolu 1 , Maddi Sridhar Reddy 2
Affiliation
|
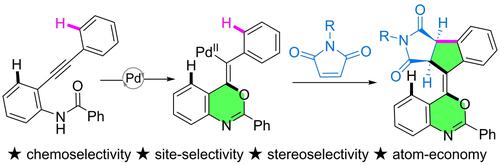
We report herein a palladium‐catalyzed, site‐selective cyclative annulation of o‐alkynyl arylamides with maleimide for the stereoselective construction of succinimide‐incorporated benzoxazine derivatives. This operationally simple and modular protocol provides access to polycyclic frameworks. The other associated features are high functional group compatibility, gram‐scale synthetic potential, and downstream transformations. Control and labeling experiments were conducted to get insights into the mechanism.
中文翻译:

(邻炔基芳基)酰胺触发的位点选择性 C−H 成环的化学选择性氧化钯化用于琥珀酰亚胺稠合多环的立体选择性合成
我们在此报道了钯催化的邻炔基芳酰胺与马来酰亚胺的位点选择性环化,用于立体选择性构建琥珀酰亚胺掺入的苯并恶嗪衍生物。这种操作简单且模块化的协议提供了对多环框架的访问。其他相关特征包括高官能团兼容性、克级合成潜力和下游转化。进行对照和标记实验以深入了解该机制。
更新日期:2024-03-19
中文翻译:

(邻炔基芳基)酰胺触发的位点选择性 C−H 成环的化学选择性氧化钯化用于琥珀酰亚胺稠合多环的立体选择性合成
我们在此报道了钯催化的邻炔基芳酰胺与马来酰亚胺的位点选择性环化,用于立体选择性构建琥珀酰亚胺掺入的苯并恶嗪衍生物。这种操作简单且模块化的协议提供了对多环框架的访问。其他相关特征包括高官能团兼容性、克级合成潜力和下游转化。进行对照和标记实验以深入了解该机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号