当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Anti-Markovnikov Selective Oxidative Arene Alkenylation Using Ir(I) Catalyst Precursors and Cu(II) Carboxylates
Organometallics ( IF 2.8 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.organomet.4c00030 Hannah Ketcham 1 , Weihao Zhu 1 , T. Brent Gunnoe 1
Organometallics ( IF 2.8 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.organomet.4c00030 Hannah Ketcham 1 , Weihao Zhu 1 , T. Brent Gunnoe 1
Affiliation
|
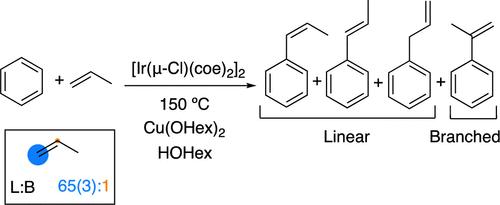
The Ir(I) complex [Ir(μ-Cl)(coe)2]2 (coe = cis-cyclooctene) is a catalyst precursor for benzene alkenylation using Cu(II) carboxylate salts. Using [Ir(μ-Cl)(coe)2]2, propenylbenzenes are formed from the reaction of benzene, propylene, and CuX2 (X = acetate, pivalate, or 2-ethylhexanoate). The Ir-catalyzed reactions selectively produce anti-Markovnikov products, trans-β-methylstyrene, cis-β-methylstyrene, and allylbenzene, along with minor amounts of the Markovnikov product, α-methylstyrene. The selectivity for the anti-Markovnikov products changed as the reaction progressed. For example, in a reaction that uses 240 equiv of Cu(OHex)2 (related to Ir), the selectivity for the anti-Markovnikov products increases from 18:1 at 3 h to 42:1 at 42 h with 30 psig of propylene at 150 °C. Studies of product stability have revealed that the increase in the selectivity for anti-Markovnikov products is not the result of an isomerization process or the selective decomposition of specific products. Rather, the change in selectivity correlates with the ratio of Cu(II) to Cu(I) in the solution, which decreases as the reaction progresses. We propose that the identity of the active catalyst changes as Cu(I) is accumulated, resulting in the formation of an active catalyst that is more selective for anti-Markovnikov products. Using a 4:1 Cu(I)/Cu(II) ratio at the start of the reaction, a 65(3):1 anti-Markovnikov/Markovnikov ratio is observed.
中文翻译:

使用 Ir(I) 催化剂前体和 Cu(II) 羧酸盐的高度抗马氏选择性氧化芳烃烯基化
Ir(I) 络合物 [Ir(μ-Cl)(coe) 2 ] 2(coe =顺式环辛烯)是使用 Cu(II) 羧酸盐进行苯烯基化的催化剂前体。使用 [Ir(μ-Cl)(coe) 2 ] 2 ,苯、丙烯和 CuX 2(X = 乙酸盐、新戊酸盐或 2-乙基己酸盐)反应形成丙烯基苯。 Ir 催化反应选择性地产生反马尔可夫尼科夫产物、反式-β-甲基苯乙烯、顺式-β-甲基苯乙烯和烯丙基苯,以及少量的马尔可夫尼科夫产物 α-甲基苯乙烯。抗马尔可夫尼科夫产物的选择性随着反应的进行而变化。例如,在使用 240 当量 Cu(OHex) 2(与 Ir 相关)的反应中,在丙烯压力为 30 psig 的情况下,抗马尔可夫尼科夫产物的选择性从 3 小时时的 18:1 增加到 42 小时时的 42:1在150°C。产品稳定性研究表明,抗马尔可夫尼科夫产物选择性的增加并不是异构化过程或特定产物选择性分解的结果。相反,选择性的变化与溶液中 Cu(II) 与 Cu(I) 的比率相关,该比率随着反应的进行而降低。我们提出,随着 Cu(I) 的积累,活性催化剂的特性发生变化,从而形成对抗马尔可夫尼科夫产物更具选择性的活性催化剂。在反应开始时使用 4:1 Cu(I)/Cu(II) 比例,观察到 65(3):1 抗马尔可夫尼科夫/马尔可夫尼科夫比例。
更新日期:2024-03-20
中文翻译:

使用 Ir(I) 催化剂前体和 Cu(II) 羧酸盐的高度抗马氏选择性氧化芳烃烯基化
Ir(I) 络合物 [Ir(μ-Cl)(coe) 2 ] 2(coe =顺式环辛烯)是使用 Cu(II) 羧酸盐进行苯烯基化的催化剂前体。使用 [Ir(μ-Cl)(coe) 2 ] 2 ,苯、丙烯和 CuX 2(X = 乙酸盐、新戊酸盐或 2-乙基己酸盐)反应形成丙烯基苯。 Ir 催化反应选择性地产生反马尔可夫尼科夫产物、反式-β-甲基苯乙烯、顺式-β-甲基苯乙烯和烯丙基苯,以及少量的马尔可夫尼科夫产物 α-甲基苯乙烯。抗马尔可夫尼科夫产物的选择性随着反应的进行而变化。例如,在使用 240 当量 Cu(OHex) 2(与 Ir 相关)的反应中,在丙烯压力为 30 psig 的情况下,抗马尔可夫尼科夫产物的选择性从 3 小时时的 18:1 增加到 42 小时时的 42:1在150°C。产品稳定性研究表明,抗马尔可夫尼科夫产物选择性的增加并不是异构化过程或特定产物选择性分解的结果。相反,选择性的变化与溶液中 Cu(II) 与 Cu(I) 的比率相关,该比率随着反应的进行而降低。我们提出,随着 Cu(I) 的积累,活性催化剂的特性发生变化,从而形成对抗马尔可夫尼科夫产物更具选择性的活性催化剂。在反应开始时使用 4:1 Cu(I)/Cu(II) 比例,观察到 65(3):1 抗马尔可夫尼科夫/马尔可夫尼科夫比例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号