当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
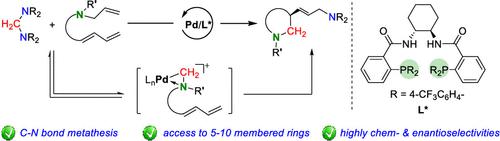
Enantioselective Ring-Closing Aminomethylamination of Allylic Aminodienes with Aminals Triggered by C–N Bond Metathesis
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.orglett.4c00641 Suchen Zou 1 , Zeyu Zhao 1 , Hanmin Huang 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.orglett.4c00641 Suchen Zou 1 , Zeyu Zhao 1 , Hanmin Huang 1, 2
Affiliation
|
A conceptually novel strategy utilizing a cyclopalladated complex as an electrophile to activate the C–N bond for the C–N bond metathesis between allylamines and aminals is developed, which enables an efficient ring-closing aminomethylamination of allylic aminodienes and aminals. The reaction proceeds under mild reaction conditions and displays a remarkable scope. Utilizing a modified Trost-type diphosphine as the ligand, this method enables the efficient synthesis of 5–10-membered aminoallylated chiral N-heterocycles in good yields with high enantiomeric excess values.
中文翻译:

C-N 键复分解引发烯丙基氨基二烯与缩醛胺的对映选择性闭环氨基甲基胺化
开发了一种概念上新颖的策略,利用环钯配合物作为亲电子试剂来激活烯丙胺和缩醛胺之间的 C-N 键复分解的 C-N 键,从而实现烯丙基氨基二烯和缩醛胺的有效闭环氨甲基胺化。该反应在温和的反应条件下进行并显示出显着的范围。该方法利用修饰的Trost型二膦作为配体,能够高效合成5-10元氨烯基化手性N-杂环,收率良好,对映体过量值高。
更新日期:2024-03-19
中文翻译:

C-N 键复分解引发烯丙基氨基二烯与缩醛胺的对映选择性闭环氨基甲基胺化
开发了一种概念上新颖的策略,利用环钯配合物作为亲电子试剂来激活烯丙胺和缩醛胺之间的 C-N 键复分解的 C-N 键,从而实现烯丙基氨基二烯和缩醛胺的有效闭环氨甲基胺化。该反应在温和的反应条件下进行并显示出显着的范围。该方法利用修饰的Trost型二膦作为配体,能够高效合成5-10元氨烯基化手性N-杂环,收率良好,对映体过量值高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号