当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
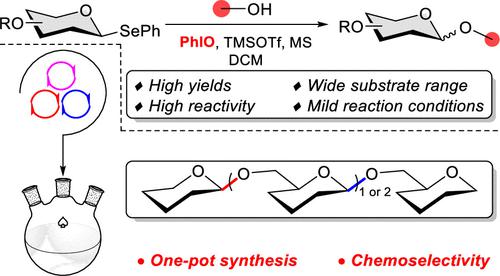
Iodosylbenzene-Promoted Glycosylation with Selenoglycosides: Application in One-Pot Glycosylation
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.orglett.4c00653 Ao Sun 1 , Ting Liu 1 , Zipeng Li 1 , Shuai Meng 2 , Xiangbao Meng 1 , Zhongtang Li 1 , Zhongjun Li 1, 3
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.orglett.4c00653 Ao Sun 1 , Ting Liu 1 , Zipeng Li 1 , Shuai Meng 2 , Xiangbao Meng 1 , Zhongtang Li 1 , Zhongjun Li 1, 3
Affiliation
|
A novel method for the glycosylation of selenoglycosides activated by iodosylbenzene was developed. The glycosylation reaction conditions were mild, fast, and efficient, with a high tolerance to diverse protecting groups and a wide substrate scope, which is advantageous for synthesizing complex glycosides. In addition, selenoglycosides were shown to be orthogonal to thioglycosides under the promotion of iodosylbenzene. Notably, a high yield of the poorly reactive glucuronidation reaction product was obtained by acetyl-protected selenoglycoside. Finally, the orthogonal one-pot synthesis of β-(1→6) oligoglucans demonstrated the usefulness of this method in oligosaccharide synthesis.
中文翻译:

碘基苯促进的硒糖苷糖基化:在一锅糖基化中的应用
开发了一种由碘酰苯激活的硒代糖苷糖基化的新方法。该糖基化反应条件温和、快速、高效,对多种保护基具有较高的耐受性,底物范围广,有利于合成复杂糖苷。此外,在碘酰苯的促进下,硒代糖苷与硫代糖苷具有正交性。值得注意的是,通过乙酰基保护的硒代糖苷获得了高产率的低反应性葡萄糖醛酸化反应产物。最后,β-(1→6)低聚糖的正交一锅法合成证明了该方法在低聚糖合成中的有用性。
更新日期:2024-03-19
中文翻译:

碘基苯促进的硒糖苷糖基化:在一锅糖基化中的应用
开发了一种由碘酰苯激活的硒代糖苷糖基化的新方法。该糖基化反应条件温和、快速、高效,对多种保护基具有较高的耐受性,底物范围广,有利于合成复杂糖苷。此外,在碘酰苯的促进下,硒代糖苷与硫代糖苷具有正交性。值得注意的是,通过乙酰基保护的硒代糖苷获得了高产率的低反应性葡萄糖醛酸化反应产物。最后,β-(1→6)低聚糖的正交一锅法合成证明了该方法在低聚糖合成中的有用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号