当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of Halogen Bonds on Protein–Peptide Binding and Protein Structural Stability Revealed by Computational Approaches
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.jmedchem.3c02359 Jintian Li 1, 2 , Liping Zhou 1, 2 , Zijian Han 1, 2 , Leyun Wu 1, 2 , Jianfang Zhang 1 , Weiliang Zhu 1, 2 , Zhijian Xu 1, 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.jmedchem.3c02359 Jintian Li 1, 2 , Liping Zhou 1, 2 , Zijian Han 1, 2 , Leyun Wu 1, 2 , Jianfang Zhang 1 , Weiliang Zhu 1, 2 , Zhijian Xu 1, 2
Affiliation
|
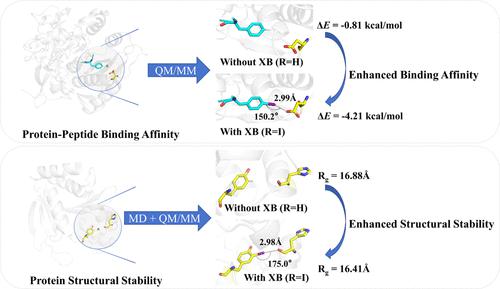
Halogen bonds (XBs) are essential noncovalent interactions in molecular recognition and drug design. Current studies on XBs in drug design mainly focus on the interactions between halogenated ligands and target proteins, lacking a systematic study of naturally existing and artificially prepared halogenated residue XBs (hr_XBs) and their characteristics. Here, we conducted a computational study on the potential hr_XBs in proteins/peptides using database searching, quantum mechanics calculations, and molecular dynamics simulations. XBs at the protein–peptide interaction interfaces are found to enhance their binding affinity. Additionally, the formation of intramolecular XBs (intra_XBs) within proteins may significantly contribute to the structural stability of structurally flexible proteins while having a minor impact on proteins with inherently high structural rigidity. Impressively, introducing halogens without the formation of intra_XBs may lead to a decrease in the protein structural stability. This study enriches our understanding of the roles and effects of halogenated residue XBs in biological systems.
中文翻译:

计算方法揭示卤素键对蛋白质-肽结合和蛋白质结构稳定性的影响
卤素键 (XB) 是分子识别和药物设计中必不可少的非共价相互作用。目前XBs在药物设计中的研究主要集中在卤代配体与靶蛋白之间的相互作用,缺乏对天然存在和人工制备的卤代残基XBs(hr_XBs)及其特性的系统研究。在这里,我们利用数据库搜索、量子力学计算和分子动力学模拟对蛋白质/肽中潜在的 hr_XB 进行了计算研究。研究发现蛋白质-肽相互作用界面上的 XB 可以增强其结合亲和力。此外,蛋白质内分子内 XB(intra_XB)的形成可能显着有助于结构柔性蛋白质的结构稳定性,同时对具有固有高结构刚性的蛋白质影响较小。令人印象深刻的是,在不形成intra_XBs的情况下引入卤素可能会导致蛋白质结构稳定性下降。这项研究丰富了我们对卤代残留物 XB 在生物系统中的作用和影响的理解。
更新日期:2024-03-19
中文翻译:

计算方法揭示卤素键对蛋白质-肽结合和蛋白质结构稳定性的影响
卤素键 (XB) 是分子识别和药物设计中必不可少的非共价相互作用。目前XBs在药物设计中的研究主要集中在卤代配体与靶蛋白之间的相互作用,缺乏对天然存在和人工制备的卤代残基XBs(hr_XBs)及其特性的系统研究。在这里,我们利用数据库搜索、量子力学计算和分子动力学模拟对蛋白质/肽中潜在的 hr_XB 进行了计算研究。研究发现蛋白质-肽相互作用界面上的 XB 可以增强其结合亲和力。此外,蛋白质内分子内 XB(intra_XB)的形成可能显着有助于结构柔性蛋白质的结构稳定性,同时对具有固有高结构刚性的蛋白质影响较小。令人印象深刻的是,在不形成intra_XBs的情况下引入卤素可能会导致蛋白质结构稳定性下降。这项研究丰富了我们对卤代残留物 XB 在生物系统中的作用和影响的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号