当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water-soluble macromers based on 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt (Na-AMPS) for rapid in situ hydrogel film formation
Polymer Chemistry ( IF 4.6 ) Pub Date : 2024-03-19 , DOI: 10.1039/d3py01416a Jinjutha Daengmankhong 1 , Sukunya Ross 1, 2 , Thanyaporn Pinthong 1 , Sararat Mahasaranon 1, 2 , Jarupa Viyoch 3 , Brian J. Tighe 4 , Matthew J. Derry 4 , Paul D. Topham 4 , Gareth Ross 1, 2
Polymer Chemistry ( IF 4.6 ) Pub Date : 2024-03-19 , DOI: 10.1039/d3py01416a Jinjutha Daengmankhong 1 , Sukunya Ross 1, 2 , Thanyaporn Pinthong 1 , Sararat Mahasaranon 1, 2 , Jarupa Viyoch 3 , Brian J. Tighe 4 , Matthew J. Derry 4 , Paul D. Topham 4 , Gareth Ross 1, 2
Affiliation
|
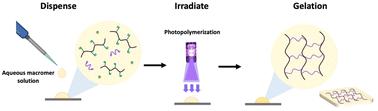
The in situ formation of hydrogels has potential for a number of biomedical applications but their generation via conventional polymerization techniques has a number of limitations, such as toxicity and reaction time. The use of macromers in hydrogel formulations can help overcome these limitations. In this work, we synthesized a new functionalized macromer formed via the copolymerization of 2-acrylamido-2-methylpropane sulfonic acid sodium salt (AMPS) and acid-functional monomers that can undergo a ring-opening reaction with allyl glycidyl ether (AGE) to generate the desired pendant vinyl macromer functionality. These macromers were characterized by 1H nuclear magnetic resonance (NMR) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy and gel permeation chromatography (GPC) to provide evidence for successful macromer synthesis and subsequent polymerization. Using a UV-initiated crosslinking approach with poly(ethylene glycol) diacrylate (PEGDA), the hydrogels were fabricated from the macromer solution, with the gelation time being reduced from 1200 s to 10 s when compared to hydrogel formation from regular vinyl monomers. While different acidic monomers result in distinct tensile properties, hydrogels containing 2-carboxyethyl acrylate (CEA) exhibit low strength but high elongation. In contrast, those with methacrylic acid (MAA) demonstrate higher strength and lower elongation. Therefore, using a balanced combination of each is a logical approach for achieving a robust final hydrogel film. In summary, we have produced a new macromer possessing characteristics highly conducive to rapid hydrogel synthesis. This macromer approach holds potential for use in in situ hydrogel formation, where a viscous solution can be applied to the target area and subsequently hardened to its hydrogel. We envisage its application primarily in the biomedical field.
中文翻译:

基于 2-丙烯酰氨基-2-甲基-1-丙磺酸钠盐 (Na-AMPS) 的水溶性大分子单体,用于快速原位水凝胶成膜
原位形成水凝胶具有许多生物医学应用的潜力,但通过传统聚合技术生成水凝胶有许多限制,例如毒性和反应时间。在水凝胶制剂中使用大分子单体可以帮助克服这些限制。在这项工作中,我们合成了一种新的功能化大分子单体,该大分子单体是通过2-丙烯酰胺基-2-甲基丙磺酸钠盐(AMPS)和酸功能单体共聚形成的,该单体可以与烯丙基缩水甘油醚(AGE)进行开环反应,生成所需的乙烯基大分子单体官能团。这些大分子单体通过1 H 核磁共振 (NMR) 光谱、傅里叶变换红外 (FT-IR) 光谱和凝胶渗透色谱 (GPC) 进行表征,为大分子单体的成功合成和后续聚合提供证据。使用聚乙二醇二丙烯酸酯 (PEGDA) 的紫外线引发交联方法,由大分子单体溶液制备水凝胶,与常规乙烯基单体形成水凝胶相比,凝胶化时间从 1200 秒缩短至 10 秒。虽然不同的酸性单体会产生不同的拉伸性能,但含有丙烯酸 2-羧乙酯 (CEA) 的水凝胶表现出低强度但高伸长率。相比之下,含有甲基丙烯酸(MAA)的材料表现出更高的强度和更低的伸长率。因此,使用每种方法的平衡组合是获得坚固的最终水凝胶膜的合理方法。总之,我们生产了一种新的大分子单体,其具有非常有利于快速水凝胶合成的特性。这种大分子单体方法具有用于原位水凝胶形成的潜力,其中可以将粘性溶液施加到目标区域并随后硬化成水凝胶。我们设想它的应用主要在生物医学领域。
更新日期:2024-03-19
中文翻译:

基于 2-丙烯酰氨基-2-甲基-1-丙磺酸钠盐 (Na-AMPS) 的水溶性大分子单体,用于快速原位水凝胶成膜
原位形成水凝胶具有许多生物医学应用的潜力,但通过传统聚合技术生成水凝胶有许多限制,例如毒性和反应时间。在水凝胶制剂中使用大分子单体可以帮助克服这些限制。在这项工作中,我们合成了一种新的功能化大分子单体,该大分子单体是通过2-丙烯酰胺基-2-甲基丙磺酸钠盐(AMPS)和酸功能单体共聚形成的,该单体可以与烯丙基缩水甘油醚(AGE)进行开环反应,生成所需的乙烯基大分子单体官能团。这些大分子单体通过1 H 核磁共振 (NMR) 光谱、傅里叶变换红外 (FT-IR) 光谱和凝胶渗透色谱 (GPC) 进行表征,为大分子单体的成功合成和后续聚合提供证据。使用聚乙二醇二丙烯酸酯 (PEGDA) 的紫外线引发交联方法,由大分子单体溶液制备水凝胶,与常规乙烯基单体形成水凝胶相比,凝胶化时间从 1200 秒缩短至 10 秒。虽然不同的酸性单体会产生不同的拉伸性能,但含有丙烯酸 2-羧乙酯 (CEA) 的水凝胶表现出低强度但高伸长率。相比之下,含有甲基丙烯酸(MAA)的材料表现出更高的强度和更低的伸长率。因此,使用每种方法的平衡组合是获得坚固的最终水凝胶膜的合理方法。总之,我们生产了一种新的大分子单体,其具有非常有利于快速水凝胶合成的特性。这种大分子单体方法具有用于原位水凝胶形成的潜力,其中可以将粘性溶液施加到目标区域并随后硬化成水凝胶。我们设想它的应用主要在生物医学领域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号