当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Fungicidal Activities of Novel N-(Pyrazol-5-yl)benzamide Derivatives Containing a Diphenylamine Moiety
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.jafc.3c07567 Yi-Dan Ma 1, 2 , Huan Zhou 2 , Guo-Tai Lin 2 , Ke-Huan Wu 2 , Gong Xu 1, 2, 3 , Xili Liu 2, 3 , Dan Xu 1, 2, 3
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.jafc.3c07567 Yi-Dan Ma 1, 2 , Huan Zhou 2 , Guo-Tai Lin 2 , Ke-Huan Wu 2 , Gong Xu 1, 2, 3 , Xili Liu 2, 3 , Dan Xu 1, 2, 3
Affiliation
|
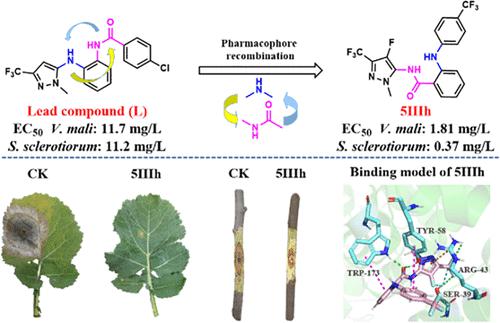
To accelerate the development of novel fungicides, a variety of N-(pyrazol-5-yl)benzamide derivatives with a diphenylamine moiety were designed and synthesized using a pharmacophore recombination strategy based on the structure of pyrazol-5-yl-aminophenyl-benzamides. The bioassay results demonstrated that most of the target compounds had excellent in vitro antifungal activities against Sclerotinia sclerotiorum, Valsa mali, and Botrytis cinerea. In particular, compound 5IIIh exhibited remarkable activity against S. sclerotiorum (EC50 = 0.37 mg/L), which was similar to that of fluxapyroxad (EC50 = 0.27 mg/L). In addition, compound 5IIIc (EC50 = 1.32 mg/L) was observed to be more effective against V. mali than fluxapyroxad (EC50 = 12.8 mg/L) and comparable to trifloxystrobin (EC50 = 1.62 mg/L). Furthermore, compound 5IIIh demonstrated remarkable in vivo protective antifungal properties against S. sclerotiorum, with an inhibition rate of 96.8% at 100 mg/L, which was close to that of fluxapyroxad (99.6%). Compounds 5IIIc (66.7%) and 5IIIh (62.9%) exhibited good in vivo antifungal effects against V. mali at 100 mg/L, which were superior to that of fluxapyroxad (11.1%) but lower than that of trifloxystrobin (88.9%). The succinate dehydrogenase (SDH) enzymatic inhibition assay was conducted to confirm the mechanism of action. Molecular docking analysis further revealed that compound 5IIIh has significant hydrogen-bonding, π–π, and p−π conjugation interactions with ARG 43, SER 39, TRP 173, and TYR 58 in the binding site of SDH, and the binding mode was similar to that of the commercial fungicide fluxapyroxad. All of the results suggest that compound 5IIIh could be a potential SDH inhibitor, offering a valuable reference for future studies.
中文翻译:

含二苯胺部分的新型 N-(吡唑-5-基)苯甲酰胺衍生物的设计、合成和杀菌活性
为了加速新型杀菌剂的开发,基于吡唑-5-基氨基苯基苯甲酰胺的结构,采用药效团重组策略设计并合成了多种带有二苯胺部分的N- (吡唑-5-基)苯甲酰胺衍生物。生物测定结果表明,大多数目标化合物对核盘菌、苹果腐烂病菌和灰葡萄孢均具有良好的体外抗真菌活性。特别是,化合物5IIIh对核盘菌表现出显着的活性(EC 50 = 0.37 mg/L),这与fluxapyroxad的活性(EC 50 = 0.27 mg/L)相似。此外,观察到化合物5IIIc (EC 50 = 1.32 mg/L)比 Fluxapyroxad (EC 50 = 12.8 mg/L)更有效对抗苹果弧菌,并且与肟菌酯 (EC 50 = 1.62 mg/L) 相当。此外,化合物5IIIh对核盘菌具有显着的体内保护性抗真菌特性,在100 mg/L浓度下的抑制率为96.8%,接近fluxapyroxad (99.6%)。化合物5IIIc(66.7%)和5IIIh(62.9%)在100 mg/L浓度下对苹果弧菌表现出良好的体内抗真菌作用,优于氟唑菌胺(11.1%),但低于肟菌酯(88.9%)。进行琥珀酸脱氢酶(SDH)酶抑制测定以确认作用机制。分子对接分析进一步表明,化合物5IIIh与SDH结合位点的ARG 43、SER 39、TRP 173、TYR 58存在显着的氢键、π-π、p-π共轭相互作用,且结合模式相似与商业杀菌剂fluxapyroxad 的效果相同。所有结果表明化合物5IIIh可能是一种潜在的SDH抑制剂,为未来的研究提供了有价值的参考。
更新日期:2024-03-18
中文翻译:

含二苯胺部分的新型 N-(吡唑-5-基)苯甲酰胺衍生物的设计、合成和杀菌活性
为了加速新型杀菌剂的开发,基于吡唑-5-基氨基苯基苯甲酰胺的结构,采用药效团重组策略设计并合成了多种带有二苯胺部分的N- (吡唑-5-基)苯甲酰胺衍生物。生物测定结果表明,大多数目标化合物对核盘菌、苹果腐烂病菌和灰葡萄孢均具有良好的体外抗真菌活性。特别是,化合物5IIIh对核盘菌表现出显着的活性(EC 50 = 0.37 mg/L),这与fluxapyroxad的活性(EC 50 = 0.27 mg/L)相似。此外,观察到化合物5IIIc (EC 50 = 1.32 mg/L)比 Fluxapyroxad (EC 50 = 12.8 mg/L)更有效对抗苹果弧菌,并且与肟菌酯 (EC 50 = 1.62 mg/L) 相当。此外,化合物5IIIh对核盘菌具有显着的体内保护性抗真菌特性,在100 mg/L浓度下的抑制率为96.8%,接近fluxapyroxad (99.6%)。化合物5IIIc(66.7%)和5IIIh(62.9%)在100 mg/L浓度下对苹果弧菌表现出良好的体内抗真菌作用,优于氟唑菌胺(11.1%),但低于肟菌酯(88.9%)。进行琥珀酸脱氢酶(SDH)酶抑制测定以确认作用机制。分子对接分析进一步表明,化合物5IIIh与SDH结合位点的ARG 43、SER 39、TRP 173、TYR 58存在显着的氢键、π-π、p-π共轭相互作用,且结合模式相似与商业杀菌剂fluxapyroxad 的效果相同。所有结果表明化合物5IIIh可能是一种潜在的SDH抑制剂,为未来的研究提供了有价值的参考。



























 京公网安备 11010802027423号
京公网安备 11010802027423号