当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalization of Alkenes with Difluoromethyl Nitrile Oxide to Access the Difluoromethylated Derivatives
Organic Letters ( IF 4.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.orglett.4c00431 Bohdan A Chalyk 1, 2, 3 , Oleksandr Zginnyk 1 , Andrii V Khutorianskyi 1 , Pavel K Mykhailiuk 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.orglett.4c00431 Bohdan A Chalyk 1, 2, 3 , Oleksandr Zginnyk 1 , Andrii V Khutorianskyi 1 , Pavel K Mykhailiuk 1
Affiliation
|
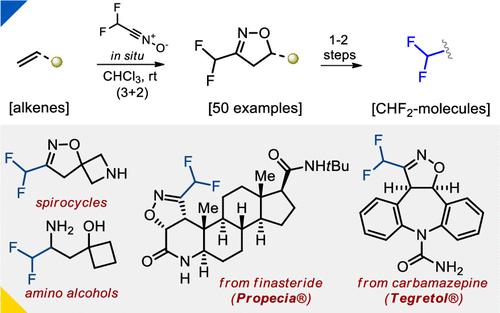
Electron-rich, electron-deficient, and non-activated alkenes can be rapidly functionalized by in situ-generated difluoromethyl nitrile oxide. The (3+2) cycloaddition proceeds at room temperature, has broad functional group tolerance, and can be used for the late-stage modification of bioactive molecules (finasteride and carbamazepine). The obtained CF2H-isoxazolines can be easily transformed into CF2H-containing building blocks for medicinal chemistry: amines, amino acids, amino alcohols, and spirocyclic scaffolds.
中文翻译:

用二氟甲基氧化腈对烯烃进行官能化以获得二氟甲基化衍生物
富电子、缺电子和未活化的烯烃可以通过原位生成的二氟甲基氧化腈快速官能化。 (3+2)环加成反应在室温下进行,具有广泛的官能团耐受性,可用于生物活性分子(非那雄胺和卡马西平)的后期修饰。所获得的CF 2 H-异恶唑啉可以很容易地转化为用于药物化学的含CF 2 H结构单元:胺、氨基酸、氨基醇和螺环支架。
更新日期:2024-03-18
中文翻译:

用二氟甲基氧化腈对烯烃进行官能化以获得二氟甲基化衍生物
富电子、缺电子和未活化的烯烃可以通过原位生成的二氟甲基氧化腈快速官能化。 (3+2)环加成反应在室温下进行,具有广泛的官能团耐受性,可用于生物活性分子(非那雄胺和卡马西平)的后期修饰。所获得的CF 2 H-异恶唑啉可以很容易地转化为用于药物化学的含CF 2 H结构单元:胺、氨基酸、氨基醇和螺环支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号