Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-17 , DOI: 10.1002/adsc.202400143 Kateřina Žáková 1 , Petra Králová 1 , Miroslav Soural 1
|
Introduction
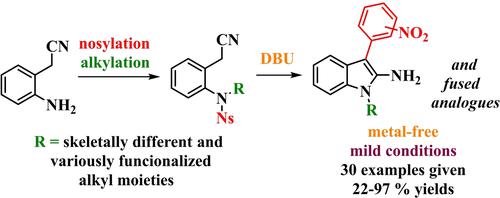
The Truce-Smiles rearrangement is an efficient strategy to form C−C bonds under metal-free conditions.1, 2 In this process, an electrophilic sp2 carbon undergoes intramolecular attack by a carbanion that originates from an activated C−H bond. The rearrangement proceeds via a 4–6 membered spiro-Meisenheimer complex, which is spontaneously converted to a C-arylated product. Recently, the Truce-Smiles rearrangement of natural amino acid-based 2/4-nitrobenzenesulfonamides was widely investigated as a route to different nitrogen heterocycles via Cα-arylation, in which C−H was typically activated by the adjacent carbonyl group (Scheme 1).3-8 Inspired by this approach, we suggested the use of 2/4-nitrobenzenesulfonamides originating from 2-aminobenzyl cyanide alkylated by scaffolds without electron-withdrawing groups. Consequently, instead of undergoing Cα-arylation, these substrates may be amenable to Cγ-arylation, which would eventually yield 2-aminoindoles.

Cα- vs. Cγ-arylation as the route to nitrogen heterocycles.
Indoles are highly relevant compounds in pharmacological fields and exhibit a broad range of biological effects.9 Although many synthetic routes leading to substituted and functionalized indoles have been reported, synthetic strategies for accessing 2-aminoindoles are rather limited. One of the oldest methods is based on the Curtius rearrangement of indolyl-2-carboxylic acid azide.10 Later, amination of the corresponding 2-nitro8 or 2-acyl12, 13 indoles to prepare 1-substituted 2-amino-3-arylindoles was reported. In alternative synthetic routes, alkynylamines have been used as the starting material for reactions with aniline-based sulfilimines14 or alkylazides.15 C−H amination of N-acyl-3-arylindo moieties via an electrooxidative cross-coupling process was developed by Feng et al.16 and the condensation of 2-(2-nitrophenyl)acetonitrile leading to N-hydroxy-2-amino-3-arylindoles was reported by Belley et al.17
To date, the most frequently studied strategies consist of rhodium-catalyzed,18 Pd-catalyzed,19 triflic acid-catalyzed17 or BF3- promoted21 arylation of 3-diazoindoline-2-imines. In this article, we report the development of a metal-free approach starting from commercially available reagents and building blocks.
中文翻译:

通过分子内 C-芳基化合成 2-氨基-3-芳基吲哚及其稠合类似物
介绍
Truce-Smiles 重排是在无金属条件下形成 C−C 键的有效策略。1, 2在此过程中,亲电sp 2 碳会受到源自活化 C−H 键的碳负离子的分子内攻击。重排通过4-6 元螺-迈森海默复合物进行,该复合物自发转化为 C-芳基化产物。最近,基于天然氨基酸的 2/4-硝基苯磺酰胺的 Truce-Smiles 重排被广泛研究,作为通过Cα-芳基化生成不同氮杂环的途径,其中 C−H 通常被相邻的羰基激活(方案 1) 。3-8受此方法的启发,我们建议使用源自 2-氨基苄氰的 2/4-硝基苯磺酰胺,并通过不含吸电子基团的支架进行烷基化。因此,这些底物可能不进行 Cα-芳基化,而是进行 Cγ-芳基化,最终产生 2-氨基吲哚。

Cα- 与 Cγ-芳基化作为氮杂环的途径。
吲哚是药理学领域高度相关的化合物,并表现出广泛的生物效应。9尽管已报道了许多产生取代和官能化吲哚的合成路线,但获得 2-氨基吲哚的合成策略相当有限。最古老的方法之一是基于吲哚基-2-甲酸叠氮化物的库尔蒂斯重排。10随后,报道了将相应的 2-硝基8或 2-酰基12, 13吲哚胺化以制备 1-取代的 2-氨基-3-芳基吲哚。在替代合成路线中,炔胺已被用作与苯胺基硫亚胺14或烷基叠氮化物反应的起始材料。 Feng等人开发了通过电氧化交叉偶联过程对N-酰基-3-芳基林基部分进行15 C−H胺化的方法。 Belley 等人报道了16和 2-(2-硝基苯基)乙腈缩合生成N-羟基-2-氨基-3-芳基吲哚。17 号
迄今为止,最常研究的策略包括铑催化、18 Pd 催化、19三氟甲磺酸催化17或 BF 3 - 促进3-重氮吲哚啉-2-亚胺的21芳基化。在本文中,我们报告了从市售试剂和构建模块开始的无金属方法的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号