当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Additive-free N-methylation reaction synergistically catalyzed by Pt single atoms and clusters on α-MoC using methanol as a sustainable C1 source
Green Chemistry ( IF 9.8 ) Pub Date : 2024-03-18 , DOI: 10.1039/d4gc00043a Shurui Fan 1, 2 , Mingyuan Zhang 3 , Xiangxin Jin 1, 2 , Zirui Gao 4 , Yao Xu 4 , Maolin Wang 4 , Chuqiao Song 1, 2 , Houhong Song 5 , Xiangxiang Chen 1, 2 , Rulong Ma 1 , Siyu Yao 5 , Rui Gao 3 , Xiaonian Li 1 , Lili Lin 1, 2
Green Chemistry ( IF 9.8 ) Pub Date : 2024-03-18 , DOI: 10.1039/d4gc00043a Shurui Fan 1, 2 , Mingyuan Zhang 3 , Xiangxin Jin 1, 2 , Zirui Gao 4 , Yao Xu 4 , Maolin Wang 4 , Chuqiao Song 1, 2 , Houhong Song 5 , Xiangxiang Chen 1, 2 , Rulong Ma 1 , Siyu Yao 5 , Rui Gao 3 , Xiaonian Li 1 , Lili Lin 1, 2
Affiliation
|
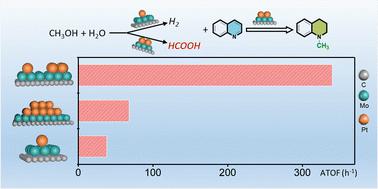
Methanol is being actively investigated as a promising C1 reagent to replace conventional C1 reagents in organic synthesis, due to its properties of being economical, abundant, nontoxic and sustainably producible. However, because of the high enthalpy of dehydrogenation, it has not been reported frequently. Herein, we report that Pt single atoms (Pt1) and Pt clusters (Ptn) are cooperatively loaded on α-MoC to achieve the conversion of various of unsaturated N-containing compounds into value-added N-methylation compounds simply in methanol aqueous solution, successfully avoiding the use of external hydrogen or any additives. The synergy between coexisting Pt1 and Ptn on Pt1+n/α-MoC is identified as necessary to realize the tandem conversion with superior activity and selectivity. Pt1/α-MoC is the active site for in situ hydrogen production from aqueous phase methanol reforming, and Ptn/α-MoC is responsible for the successive hydrogenation and methylation. The partially positive Ptnδ+ species are crucial for the reductive N-methylation reaction, as the strong coordination of N-containing compounds to Pt particles (Ptn) significantly inhibits the catalytic activity. Experimental and DFT results further show that, unlike the traditional hydrogen-borrowing mechanism of methanol via the intermediate formaldehyde, formic acid is found to be a potent intermediate and agent for N-methylation.
中文翻译:

使用甲醇作为可持续的 C1 源,α-MoC 上的 Pt 单原子和簇协同催化无添加剂的 N-甲基化反应
甲醇由于其经济、丰富、无毒和可持续生产的特性,正在被积极研究作为一种有前景的 C1 试剂,以取代有机合成中的传统 C1 试剂。但由于脱氢反应的热焓较高,目前尚未见报道。在此,我们报道了Pt单原子(Pt 1)和Pt簇(Pt n)协同负载在α-MoC上,实现了多种不饱和含氮化合物在甲醇水溶液中简单地转化为增值的N-甲基化化合物解决方案,成功避免使用外部氢气或任何添加剂。Pt 1+ n /α-MoC 上共存的 Pt 1和 Pt n之间的协同作用被认为是实现具有优异活性和选择性的串联转化所必需的。 Pt 1 /α-MoC是水相甲醇重整原位制氢的活性位点,Pt n /α-MoC负责连续的氢化和甲基化。部分正电的 Pt n δ +物种对于还原性N甲基化反应至关重要,因为含氮化合物与 Pt 颗粒 (Pt n )的强配位显着抑制催化活性。实验和DFT结果进一步表明,与传统的甲醇通过中间体甲醛借氢机制不同,甲酸是一种有效的中间体和N-甲基化试剂。
更新日期:2024-03-18
中文翻译:

使用甲醇作为可持续的 C1 源,α-MoC 上的 Pt 单原子和簇协同催化无添加剂的 N-甲基化反应
甲醇由于其经济、丰富、无毒和可持续生产的特性,正在被积极研究作为一种有前景的 C1 试剂,以取代有机合成中的传统 C1 试剂。但由于脱氢反应的热焓较高,目前尚未见报道。在此,我们报道了Pt单原子(Pt 1)和Pt簇(Pt n)协同负载在α-MoC上,实现了多种不饱和含氮化合物在甲醇水溶液中简单地转化为增值的N-甲基化化合物解决方案,成功避免使用外部氢气或任何添加剂。Pt 1+ n /α-MoC 上共存的 Pt 1和 Pt n之间的协同作用被认为是实现具有优异活性和选择性的串联转化所必需的。 Pt 1 /α-MoC是水相甲醇重整原位制氢的活性位点,Pt n /α-MoC负责连续的氢化和甲基化。部分正电的 Pt n δ +物种对于还原性N甲基化反应至关重要,因为含氮化合物与 Pt 颗粒 (Pt n )的强配位显着抑制催化活性。实验和DFT结果进一步表明,与传统的甲醇通过中间体甲醛借氢机制不同,甲酸是一种有效的中间体和N-甲基化试剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号