当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigations on Superbase Mediated Reactivity of N-Tosylhydrazones with Aza-ortho-Quinone Methide Precursors
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-03-15 , DOI: 10.1002/ejoc.202400151 Rahul P. 1 , Arunkumar T. K. 1 , Seena Sebastian 1 , Haritha Raveendran 1 , Sunil Varughese 1 , Jomon Mathew 2 , Jubi John 3
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-03-15 , DOI: 10.1002/ejoc.202400151 Rahul P. 1 , Arunkumar T. K. 1 , Seena Sebastian 1 , Haritha Raveendran 1 , Sunil Varughese 1 , Jomon Mathew 2 , Jubi John 3
Affiliation
|
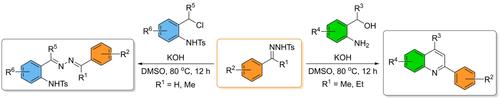
Investigations were made on the chemoselectivity in superbase-mediated reactions of N-tosylhydrazones with aza-ortho-quinone methide precursors. Under super basic conditions, the reaction of N-tosylhydrazone with ortho-aminobenzyl afforded 2-substituted quinoline while the reaction of former with N-(2-(chloromethyl)phenyl)-4-methylbenzenesulfonamide furnished azine sulphonamides.
中文翻译:

N-甲苯磺酰腙与氮杂邻醌甲基化物前体的超碱介导反应性研究
对N-甲苯磺酰腙与氮杂邻醌甲基化物前体的超碱介导反应的化学选择性进行了研究。在超碱性条件下, N-甲苯磺酰腙与邻氨基苄基反应生成2-取代喹啉,N-甲苯磺酰腙与N- (2-(氯甲基)苯基)-4-甲基苯磺酰胺反应生成吖嗪磺酰胺。
更新日期:2024-03-15
中文翻译:

N-甲苯磺酰腙与氮杂邻醌甲基化物前体的超碱介导反应性研究
对N-甲苯磺酰腙与氮杂邻醌甲基化物前体的超碱介导反应的化学选择性进行了研究。在超碱性条件下, N-甲苯磺酰腙与邻氨基苄基反应生成2-取代喹啉,N-甲苯磺酰腙与N- (2-(氯甲基)苯基)-4-甲基苯磺酰胺反应生成吖嗪磺酰胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号