当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Bufadienolide Cinobufagin via Late-Stage Singlet Oxygen Oxidation/Rearrangement Approach
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.orglett.4c00625 Colin Tichvon 1 , Eugene Zviagin 1 , Zoey Surma 1 , Pavel Nagorny 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.orglett.4c00625 Colin Tichvon 1 , Eugene Zviagin 1 , Zoey Surma 1 , Pavel Nagorny 1
Affiliation
|
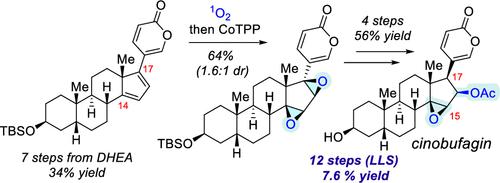
This manuscript describes a concise synthesis of cinobufagin, a natural steroid of the bufadienolide family, from readily available dehydroepiandrosterone (DHEA), as well as its α5-epimer derived from 3-epi-andosterone. This synthesis features expedient installation of the 17β-pyrone moiety with the 14β,15β-epoxide and the 16β-acetoxy group using a photochemical regioselective singlet oxygen [4 + 2] cycloaddition followed by CoTPP-promoted in situ endoperoxide rearrangement to provide a 14β,16β-bis-epoxide in 64% yield with a 1.6:1 d.r. This β,β-bis-epoxide intermediate was subsequently subjected to a regioselective scandium(III) trifluoromethanesulfonate catalyzed House–Meinwald rearrangement to establish the 17β-configuration. The synthesis of cinobufagin is achieved in 12 steps (LLS) and 7.6% overall yield, and we demonstrate that it could be used as a platform for the subsequent medicinal chemistry exploration of cinobufagin analogs such as cinobufagin 5α-epimer.
中文翻译:

通过后期单线态氧氧化/重排方法合成蟾蜍二烯内酯华蟾素精
该手稿描述了华蟾蜍精(蟾蜍二烯内酯家族的一种天然类固醇)的简明合成,其由容易获得的脱氢表雄酮(DHEA)及其衍生自 3-表雄酮的 α5-差向异构体组成。该合成的特点是使用光化学区域选择性单线态氧 [4 + 2] 环加成,将 17β-吡喃酮部分与 14β,15β-环氧化物和 16β-乙酰氧基方便地安装在一起,然后通过 CoTPP 促进的原位内过氧化物重排来提供 14β, 16β-双环氧化物,产率 64%,dr 为 1.6:1 这种 β,β-双环氧化物中间体随后进行区域选择性三氟甲磺酸钪 (III) 催化的 House-Meinwald 重排,以建立 17β-构型。华蟾素的合成经过12步(LLS)实现,总产率为7.6%,我们证明它可以作为华蟾素类似物(如华蟾素5α-差向异构体)后续药物化学探索的平台。
更新日期:2024-03-15
中文翻译:

通过后期单线态氧氧化/重排方法合成蟾蜍二烯内酯华蟾素精
该手稿描述了华蟾蜍精(蟾蜍二烯内酯家族的一种天然类固醇)的简明合成,其由容易获得的脱氢表雄酮(DHEA)及其衍生自 3-表雄酮的 α5-差向异构体组成。该合成的特点是使用光化学区域选择性单线态氧 [4 + 2] 环加成,将 17β-吡喃酮部分与 14β,15β-环氧化物和 16β-乙酰氧基方便地安装在一起,然后通过 CoTPP 促进的原位内过氧化物重排来提供 14β, 16β-双环氧化物,产率 64%,dr 为 1.6:1 这种 β,β-双环氧化物中间体随后进行区域选择性三氟甲磺酸钪 (III) 催化的 House-Meinwald 重排,以建立 17β-构型。华蟾素的合成经过12步(LLS)实现,总产率为7.6%,我们证明它可以作为华蟾素类似物(如华蟾素5α-差向异构体)后续药物化学探索的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号