当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A radical 1,4-aryl migration enables nickel-catalysed remote cross-electrophile coupling of β-bromo amino acid esters with vinyl triflates
Chemical Communications ( IF 4.9 ) Pub Date : 2024-03-15 , DOI: 10.1039/d4cc00627e Yin-Ling Liu 1 , Jian Liu 1 , Xin-Yu Li 1 , Peng He 1 , Yu-Xuan Liu 1 , Mei Xiang 1 , Shi Tang 1
Chemical Communications ( IF 4.9 ) Pub Date : 2024-03-15 , DOI: 10.1039/d4cc00627e Yin-Ling Liu 1 , Jian Liu 1 , Xin-Yu Li 1 , Peng He 1 , Yu-Xuan Liu 1 , Mei Xiang 1 , Shi Tang 1
Affiliation
|
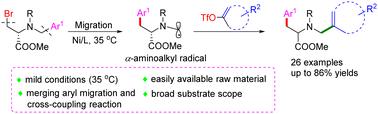
A radical 1,4-aryl migration enabling a cross-electrophile coupling reaction toward remote transalkylation of N-benzyl alanine has been developed. In this strategy, with the occurrence of a radical-mediated Turce-Smiles rearrangement, key α-aminoalkyl radicals are generated. The as-formed α-aminoalkyl radical serves as a robust coupling partner for cross-electrophilic coupling with vinyl triflates, affording a series of olefin-tethered amino acid motifs.
中文翻译:

自由基 1,4-芳基迁移使得镍催化的 β-溴氨基酸酯与三氟甲磺酸乙烯酯发生远程交叉亲电子偶联
已经开发出一种自由基 1,4-芳基迁移,能够实现N-苄基丙氨酸远程反烷基化的交叉亲电子偶联反应。在该策略中,随着自由基介导的 Turce-Smiles 重排的发生,产生了关键的 α-氨基烷基自由基。所形成的α-氨基烷基作为与三氟甲磺酸乙烯酯交叉亲电偶联的稳健偶联伙伴,提供一系列烯烃束缚的氨基酸基序。
更新日期:2024-03-15
中文翻译:

自由基 1,4-芳基迁移使得镍催化的 β-溴氨基酸酯与三氟甲磺酸乙烯酯发生远程交叉亲电子偶联
已经开发出一种自由基 1,4-芳基迁移,能够实现N-苄基丙氨酸远程反烷基化的交叉亲电子偶联反应。在该策略中,随着自由基介导的 Turce-Smiles 重排的发生,产生了关键的 α-氨基烷基自由基。所形成的α-氨基烷基作为与三氟甲磺酸乙烯酯交叉亲电偶联的稳健偶联伙伴,提供一系列烯烃束缚的氨基酸基序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号