当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting Mycobacterium tuberculosis Persistence through Inhibition of the Trehalose Catalytic Shift
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2024-03-14 , DOI: 10.1021/acsinfecdis.4c00138 Karishma Kalera 1, 2 , Rachel Liu 3 , Juhyeon Lim 3 , Rasangi Pathirage 4 , Daniel H. Swanson 1 , Ulysses G. Johnson 1, 2 , Alicyn I. Stothard 1 , Jae Jin Lee 3 , Anne W. Poston 1 , Peter J. Woodruff 5 , Donald R. Ronning 4 , Hyungjin Eoh 3 , Benjamin M. Swarts 1, 2
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2024-03-14 , DOI: 10.1021/acsinfecdis.4c00138 Karishma Kalera 1, 2 , Rachel Liu 3 , Juhyeon Lim 3 , Rasangi Pathirage 4 , Daniel H. Swanson 1 , Ulysses G. Johnson 1, 2 , Alicyn I. Stothard 1 , Jae Jin Lee 3 , Anne W. Poston 1 , Peter J. Woodruff 5 , Donald R. Ronning 4 , Hyungjin Eoh 3 , Benjamin M. Swarts 1, 2
Affiliation
|
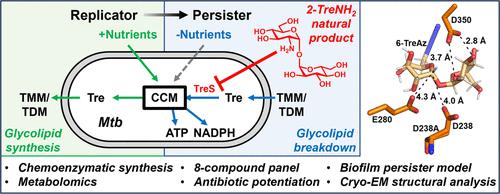
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is the leading cause of death worldwide by infectious disease. Treatment of Mtb infection requires a six-month course of multiple antibiotics, an extremely challenging regimen necessitated by Mtb’s ability to form drug-tolerant persister cells. Mtb persister formation is dependent on the trehalose catalytic shift, a stress-responsive metabolic remodeling mechanism in which the disaccharide trehalose is liberated from cell surface glycolipids and repurposed as an internal carbon source to meet energy and redox demands. Here, using a biofilm-persister model, metabolomics, and cryo-electron microscopy (EM), we found that azidodeoxy- and aminodeoxy-d-trehalose analogues block the Mtb trehalose catalytic shift through inhibition of trehalose synthase TreS (Rv0126), which catalyzes the isomerization of trehalose to maltose. Out of a focused eight-member compound panel constructed by chemoenzymatic synthesis, the natural product 2-trehalosamine exhibited the highest potency and significantly potentiated first- and second-line TB drugs in broth culture and macrophage infection assays. We also report the first structure of TreS bound to a substrate analogue inhibitor, obtained via cryo-EM, which revealed conformational changes likely essential for catalysis and inhibitor binding that can potentially be exploited for future therapeutic development. Our results demonstrate that inhibition of the trehalose catalytic shift is a viable strategy to target Mtb persisters and advance trehalose analogues as tools and potential adjunctive therapeutics for investigating and targeting mycobacterial persistence.
中文翻译:

通过抑制海藻糖催化转变来靶向结核分枝杆菌的持久性
结核病 (TB) 由结核分枝杆菌(Mtb)引起,是全世界传染病死亡的主要原因。结核分枝杆菌感染的治疗需要六个月的多种抗生素疗程,这是一种极具挑战性的治疗方案,因为结核分枝杆菌具有形成耐药持久细胞的能力。结核分枝杆菌持续细胞的形成依赖于海藻糖催化转变,这是一种应激响应性代谢重塑机制,其中二糖海藻糖从细胞表面糖脂中释放出来,并重新用作内部碳源以满足能量和氧化还原需求。在这里,使用生物膜持久模型、代谢组学和冷冻电子显微镜 (EM),我们发现叠氮脱氧-和氨基脱氧-d-海藻糖类似物通过抑制海藻糖合酶 TreS (Rv0126) 来阻断 Mtb 海藻糖催化转变,TreS 催化海藻糖异构化为麦芽糖。在通过化学酶合成构建的集中八元化合物组中,天然产物 2-海藻糖胺在肉汤培养和巨噬细胞感染测定中表现出最高效力且显着增强的一线和二线结核病药物。我们还报告了通过冷冻电镜获得的 TreS 与底物类似物抑制剂结合的第一个结构,该结构揭示了可能对于催化和抑制剂结合至关重要的构象变化,这些变化可能可用于未来的治疗开发。我们的结果表明,抑制海藻糖催化转变是针对结核分枝杆菌持久性的可行策略,并推进海藻糖类似物作为研究和靶向分枝杆菌持久性的工具和潜在辅助疗法。
更新日期:2024-03-14
中文翻译:

通过抑制海藻糖催化转变来靶向结核分枝杆菌的持久性
结核病 (TB) 由结核分枝杆菌(Mtb)引起,是全世界传染病死亡的主要原因。结核分枝杆菌感染的治疗需要六个月的多种抗生素疗程,这是一种极具挑战性的治疗方案,因为结核分枝杆菌具有形成耐药持久细胞的能力。结核分枝杆菌持续细胞的形成依赖于海藻糖催化转变,这是一种应激响应性代谢重塑机制,其中二糖海藻糖从细胞表面糖脂中释放出来,并重新用作内部碳源以满足能量和氧化还原需求。在这里,使用生物膜持久模型、代谢组学和冷冻电子显微镜 (EM),我们发现叠氮脱氧-和氨基脱氧-d-海藻糖类似物通过抑制海藻糖合酶 TreS (Rv0126) 来阻断 Mtb 海藻糖催化转变,TreS 催化海藻糖异构化为麦芽糖。在通过化学酶合成构建的集中八元化合物组中,天然产物 2-海藻糖胺在肉汤培养和巨噬细胞感染测定中表现出最高效力且显着增强的一线和二线结核病药物。我们还报告了通过冷冻电镜获得的 TreS 与底物类似物抑制剂结合的第一个结构,该结构揭示了可能对于催化和抑制剂结合至关重要的构象变化,这些变化可能可用于未来的治疗开发。我们的结果表明,抑制海藻糖催化转变是针对结核分枝杆菌持久性的可行策略,并推进海藻糖类似物作为研究和靶向分枝杆菌持久性的工具和潜在辅助疗法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号