当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed 1,2-Alkynylarylation of Vinyl Arenes with Haloalkynes and Arylboronic Acids
Organic Letters ( IF 4.9 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.orglett.4c00051 Ruize Ma 1 , Songjia Fang 1 , Huanfeng Jiang 1 , Wanqing Wu 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.orglett.4c00051 Ruize Ma 1 , Songjia Fang 1 , Huanfeng Jiang 1 , Wanqing Wu 1
Affiliation
|
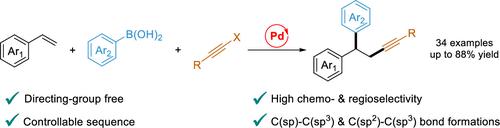
We herein disclose a novel palladium-catalyzed 1,2-alkynylarylation of vinyl arenes using haloalkynes and arylboronic acids as coupling partners. This reaction is characterized by broad substrate scope, controllable reaction sequence, and excellent chemo- and regioselectivities. Mechanistic investigations suggest that the reaction is initiated by regioselective insertion of vinyl arenes into the alkynyl-Pd(II) species, and the silver salt is crucial for this transformation, serving as both the Lewis acid and halide scavenger. This protocol provides efficient access to new carbon skeletons, which are embedded in the key biologically active motifs.
中文翻译:

钯催化乙烯基芳烃与卤代炔和芳基硼酸的 1,2-炔基芳基化
我们在此公开了使用卤代炔和芳基硼酸作为偶联配偶体的新型钯催化的乙烯基芳烃的1,2-炔基芳基化。该反应具有底物范围广、反应顺序可控以及优异的化学和区域选择性的特点。机理研究表明,该反应是通过将乙烯基芳烃区域选择性插入到炔基-Pd(II)物种中而引发的,而银盐对于这种转变至关重要,充当路易斯酸和卤化物清除剂。该协议提供了对新碳骨架的有效访问,这些骨架嵌入了关键的生物活性图案中。
更新日期:2024-03-15
中文翻译:

钯催化乙烯基芳烃与卤代炔和芳基硼酸的 1,2-炔基芳基化
我们在此公开了使用卤代炔和芳基硼酸作为偶联配偶体的新型钯催化的乙烯基芳烃的1,2-炔基芳基化。该反应具有底物范围广、反应顺序可控以及优异的化学和区域选择性的特点。机理研究表明,该反应是通过将乙烯基芳烃区域选择性插入到炔基-Pd(II)物种中而引发的,而银盐对于这种转变至关重要,充当路易斯酸和卤化物清除剂。该协议提供了对新碳骨架的有效访问,这些骨架嵌入了关键的生物活性图案中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号